Impact of Solvent Impurities on Stainless Steel Corrosion in CO2-Saturated NaCl Environments: Mechanisms and Implications
Department of Chemistry, Sri Krishna College of Technology, Coimbatore, Tamil Nadu, India.
Corresponding Author E-mail: k.r.kanimozhi@skct.edu.in
DOI : http://dx.doi.org/10.13005/ojc/400121
Article Received on : 22 Nov 2023
Article Accepted on :
Article Published : 15 Feb 2024
Reviewed by: Dr. K.R. Kanimozhi
Second Review by: Dr. Hojat Jafari
Final Approval by: Dr. Ayssar Nahle
The presence of solvent impurities can significantly impact the corrosion performance of stainless steel when exposed to CO2-saturated NaCl (Sodium Chloride) solutions. Solvent impurities, originating from various sources, may introduce chemical changes that interact with the metal surface and alter its corrosion resistance. Under simulated flow circumstances, the corrosion performance of stainless steels in the presence of contaminants such Monoethyleneglycol (MEG) and oxygen in a CO2 environment was investigated. Utilizing a rotating cage, the corrosion performance of stainless steel was assessed, and mass loss measurements were used to calculate the corrosion rates. According to the experimental findings, the oxygenated MEG exhibits a very low rate of corrosion. SEM and EDX surface analysis techniques were utilized to examine the corrosion product that had developed on the metal surface.
KEYWORDS:Carbon Capture; CO2 sequestration; CO2 Corrosion; MEG; Rotating Cage (RC); Sequestration network; SS corrosion
Download this article as:| Copy the following to cite this article: Kanimozhi K. R. Impact of Solvent Impurities on Stainless Steel Corrosion in CO2-Saturated NaCl Environments: Mechanisms and Implications. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Kanimozhi K. R. Impact of Solvent Impurities on Stainless Steel Corrosion in CO2-Saturated NaCl Environments: Mechanisms and Implications. Orient J Chem 2024;40(1). Available from: https://bit.ly/42Gj4Z2 |
Introduction
The Industrial Revolution marked a significant turning point in human history, characterized by the widespread adoption of mechanized production processes, which relied heavily on fossil fuels such as coal and later oil. These fuels release carbon dioxide (CO2) and other greenhouse gases (GHGs) when burned, leading to an increase in atmospheric CO2 levels. This has contributed to the phenomenon of global warming and climate change. The heightened levels of CO2 and other GHGs in the atmosphere have led to a warming of the planet, resulting in various environmental and climatic changes. Human activities, primarily the burning of fossil fuels for energy, deforestation, and certain industrial processes, are responsible for the majority of the CO2 emissions driving climate change. These anthropogenic activities have disrupted the natural balance of carbon cycling, leading to an accumulation of CO2 in the atmosphere. While renewable energy sources such as solar, wind, hydroelectric, and geothermal are being increasingly adopted to mitigate carbon emissions, the transition away from fossil fuels is a complex and gradual process. Fossil fuels still dominate the global energy landscape due to their convenience and high energy density. CCS is often considered a bridging technology because it allows us to continue using fossil fuels for energy while simultaneously reducing emissions. This can help us to meet immediate energy demands while transitioning towards a low-carbon or carbon-neutral energy future1. The fundamental concept behind CCS is to carefully transport and store CO2 from large industrial sources in developed oil and gas reservoirs or other deep geological formations like saline aquifers where it would stay for thousands of years or longer. CO2 Corrosion is one of the main issues with CO2 collecting and transportation. The most flexible approach for removing CO2 from industrial exhaust gas is the gas absorption technique, which uses aqueous alkanolamine solutions; this process is also referred to as the amine treatment procedure. All amine treatment facilities have, nevertheless, experienced corrosion problems2. Studies have shown that the temperature and purity of the flue gas, along with the materials used to construct the installation, have a significant impact on the rate of corrosion, which has long been considered one of the most serious operating issues in alkanolamine power plants.3 Industrial materials that are often utilized are inert to dry CO2. However, the acid gas CO2 will combine with water to generate the corrosive carbonic acid, which is corrosive. Carbonic acid corrosion, also known as “sweet gas corrosion”, is a well-known and significant issue in the oil and gas industry, particularly in pipelines that transport hydrocarbon gases containing carbon dioxide (CO2) and hydrogen sulfide (H2S).4
The feasible and best immediate solution for CO2 capture from power plants is the chemical absorption of CO2. By running the flue gas through a scrubbing system made up of an absorber and a desorber, CO2 is removed from the flue gas. CO2 and an aqueous alkaline solvent undergo a reversible chemical reaction as part of the absorption process. The desorber separates the absorbed CO2 from the solution and sends a pure stream of compressed CO2 while returning the regenerated solvent to the absorber 5,6.
Physical solvent scrubbing of CO2 with glycol is a well-established method. When there is a highly concentrated stream of CO2 at high pressures, physical solvents are used. Since many years ago, monoethylene glycol (MEG), a glycol-based solvent, has been used to process natural gas. It is effective for capturing both CO2 and H2S at greater concentrations as well as removing CO2 in bulk. 7
Depending on the method and capture technology, the CO2 taken from various sources may contain a wide range of contaminants. The behavior of the gas in the pipeline may significantly change as a result of CO2 contamination, which could have severe repercussions. In addition to this, impurities like NOx, SOx, and O2, CO2 stream may also contain small amounts of chemical solvents (amine based solvents) used in the post-combustion capture process.8,9. MEG (solvent), which might act as an impurity, could enter the pipeline used to transport CO2 during the scrubbing processes.
Despite the fact that CO2 is transported under supercritical conditions, aqueous water phase develops during the shutdown period, either accidentally or as a result of operational needs. Additionally, highly turbulent flows are generated when CO2 is injected into saline subterranean water, which favors flow-induced localized corrosion (FILC). 10
By comprehensively examining the impact of solvent impurities on stainless steel corrosion in CO2 – saturated NaCl solutions, this study contributes to a deeper understanding of corrosion mechanisms and aids in the development of effective corrosion prevention and mitigation strategies for industrial applications.
Although we currently know little about how impurities affect material properties, we must choose the right materials for the CO2 sequestration network, the corrosion performance of stainless steel in atmospheric pressure under flow conditions and the influence of impurities like ethylene glycol were investigated. Research studies of the impact of dissolved oxygen on the corrosion of stainless steel in 4M MEG were also conducted through experiments.
Therefore, the primary objective of this investigation is to analyze the specific influence of such solvent impurities on stainless steel in a CO2 rich and saline environment.
Experimental Methods and Materials
Preparation of samples
The samples used in this study consist of two distinct types of stainless steels, specifically 304SS and 316SS. These samples were provided by CANMET Materials and Technology, Canada and were of dimensions 7.5×1.9×0.3cm.
To prepare the samples for the experimental study, the received metal specimens were polished using high-quality silicon carbide (SiC) emery sheets with a grit size of 400. Once the polishing procedure was completed, the specimens were subjected to a thorough washing using distilled water. Following the washing step, the samples were carefully dried and subsequently stored in the desiccators. These desiccators were employed to maintain a controlled and dry environment, ensuring the samples readiness for use in the subsequent phases of the study.
Preparation of Reagents
The test media used for the present investigation were prepared in deionised water and a constant volume of four litres were used. The composition of the test media encompassed various solutions of 4 M MEG (Monoethylene Glycol) combined with different concentrations of NaCl (Sodium chloride) of 1,5,15 and 30% concentrations.
For the specific investigation involving the 4M MEG solution, an additional factor was introduced to replicate real-world conditions. This involved the inclusion of an impurity in the form of oxygen. To achieve this, the test solution was subjected to an oxygenation process lasting four hours. Subsequently, the solution was further treated by being pre-saturated with CO2 for duration of four hours. This pre-saturation process was executed at a controlled rate of four bubbles per second. It’s important to note that the procedures adhered to the guidelines outlined in ASTM G 202, ensuring a standardized and consistent approach throughout the reagent preparation stage.
Characterization Methods
All the experiments were carried out with the above test media using the atmospheric Rotating Cage which is a simple, compact methodology to stimulate pipeline flow conditions in the laboratory to evaluate the corrosion of all the metals 11. ASTM G170, G184-06 and G202 provide the standard practice for using rotating cage The rotating cage’s setup and schematic illustration are shown in Figures 1(a), (b), and (c).
 |
Figure 1(a): Schematic illustration of the rotating cage. |
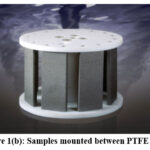 |
Figure 1(b): Samples mounted between PTFE disks. |
 |
Figure 1(c): Rotating cage at 500 rpm Corrosion Rate |
Corrosion Rate
The mass loss was calculated, and three specimens’ average results were given. The following formula was used to determine the corrosion rate in accordance with ASTM G1 standard.
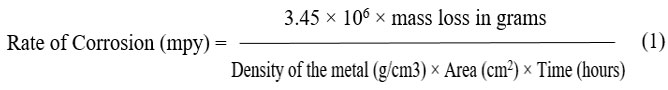
Results and discussion
Effect of MEG on CO2 Corrosion
Monoethylene glycol (MEG) is primarily utilized in gas treatment systems, as well as in wet pipelines for natural gas running at low temperatures to prevent the formation of solid gas hydrates. On CO2 corrosion, glycols have a marginally inhibitory effect. Since the glycol would be exposed to air during handling and transportation, injecting oxygen-containing glycol there could result in corrosion issues.12,13
To investigate this phenomenon, a series of mass loss experiments were conducted. These studies were created to investigate the effects of dissolved oxygen (O2) on the corrosion of stainless steel in MEG solutions that are CO2-saturated. To facilitate these investigations, an atmospheric rotating cage setup was employed, operating at a consistent speed of 500 rotations per minute (rpm). The experiments aimed to assess the influence of NaCl solution at various concentrations in conjunction with 4M MEG, which served as a solvent impurity. The assessments were conducted over different time intervals of rotation.
By employing this experimental setup and methodology, we can gain a deeper understanding of the potential corrosion effects arising from the interaction of oxygen-containing glycol with CO2 within MEG solutions. The results of these experiments could provide valuable insights into corrosion prevention strategies for stainless steel components within pipeline systems. The mean corrosion rates are offered in the Tables 1 and 2.
Table 1: Mean 304 SS rate of corrosion in NaCl with 4M MEG as an Impurity. Flow condition: 500rpm
|
S. No |
Period of Rotation |
Mean Corrosion rate (mpy) |
|||
|
1% (w/v) NaCl + 4M MEG |
5% (w/v) NaCl + 4M MEG |
15% (w/v) NaCl + 4M MEG |
30% (w/v) NaCl + 4M MEG |
||
|
1 |
24 h |
2.81796 |
4.59052 |
3.07931 |
1.09536 |
|
2 |
48 h |
0.93695 |
0.60181 |
0.36358 |
0.47203 |
|
3 |
72 h |
0.55944 |
0.14105 |
0.51321 |
0.31646 |
|
4 |
96 h |
0.30224 |
0.09867 |
0.53159 |
0.27735 |
Table 2: Mean 316 SS rate of corrosion in NaCl with 4M MEG as an impurity Flow condition: 500rpm.
|
S. No |
Period of Rotation |
Mean Corrosion rate (mpy) |
|||
|
1% (w/v) NaCl + 4M MEG |
5% (w/v) NaCl + 4M MEG |
15% (w/v) NaCl + 4M MEG |
30% (w/v) NaCl + 4M MEG |
||
|
1 |
24 h |
0.36618 |
1.35858 |
0.54662 |
1.04547 |
|
2 |
48 h |
0.20874 |
0.10879 |
0.43428 |
0.83053 |
|
3 |
72 h |
0.08491 |
0.06781 |
0.63093 |
0.74297 |
|
4 |
96 h |
0.05828 |
0.19547 |
1.05873 |
0.47541 |
The impact of NaCl and rotation period on stainless steel with 4M MEG
The effect of NaCl content and rotation time on stainless steel behavior in the presence of 4M MEG (Monoethylene Glycol) is significant. Initially, the corrosion rate is relatively high, but as the reaction a progress, the rate of corrosion diminishes. The production of corrosion products, which acts as a barrier to avoid direct contact between the metal surface and corrosive ions, is responsible for this decrease in rate of corrosion 14,15,16. Consequently, the corrosion process becomes hindered by this protective layer.
Stainless steel exhibits minimal weight loss due to pitting or localized attack. There is a localized disruption of the passive film that typically covers the metal surface. The presence of halide ions, particularly chloride ions, contributes to the initiation and progression of localized corrosion. A pit that has already formed may turn into a crevice. 17, further exacerbating the corrosion process. 18,19,20,21
The most supporting factor for the decrease in the corrosion of stainless steels in NaCl solution with MEG is due to the to the testing medium’s high pH value. The test media’s measured pH falls between 9.5 and 10.8, this is high enough to behave as an alkaline solution. This alkaline environment likely contributes to the observed reduction in corrosion rates.
Comparative analysis of the mean corrosion rates of the selected stainless steel types in NaCl solution with MEG, across different durations of rotation, reveals a distinct corrosion behaviour order. Specifically, the corrosion behaviour of these metals found to follow the order:
304 SS > 316 SS.
This order implies that 304 stainless steel exhibits a higher susceptibility to corrosion compared to 316 stainless steel under the given experimental settings.
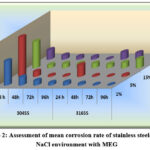 |
Figure 2: Assessment of mean corrosion rate of stainless steels under NaCl environment with MEG |
Surface Analysis
Stainless steels exposed to 30% NaCl with 4M MEG for 96 h showed higher corrosion rate. Upon inspection, the surface of the samples exhibited complete coverage by corrosion products, accompanied by minor instances of pitting. The SEM image and the corresponding EDX graphs are presented in figures 3 and 4 respectively.
From the analysis, it is understood that the formation of layer may be due to the presence of high molybdenum content present in the stainless steel, which prevents crevice and pit corrosion, and high nickel content prevents chloride-ion stress corrosion cracking 22. This molybdenum content plays a role in mitigating pitting and crevice corrosion. Additionally, the high nickel content in the steel contributes to its resistance against stress corrosion cracking due to Chloride ion content. The corrosion confrontation of stainless steel may be due to the passive film formation by chromium and molybdenum. The chromium-stabilized film is thought to be continuous, nonporous, insoluble, passive, and self-healing for any stainless steel 23,24. This passive oxide film, primarily Cr2O3, is instrumental in minimizing the corrosive actions on the metal surface.
However, it’s important to note that while the oxide layer imparts passivity to the material, the presence of cracks within this protective layer can render the metal surface active. This shift towards an active state can lead to the progression of cracks and subsequently continue the corrosion process. In essence, while the oxide layer aims to provide protection, the presence of cracks can compromise its effectiveness and allow for corrosion.
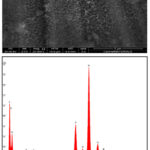 |
Figure 3: SEM image and EDX spectra of 304 SS in NaCl (30%) with MEG at 96 hours |
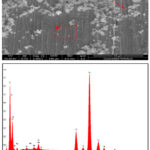 |
Figure 4: SEM image and EDX spectra of 316 SS in NaCl (30%) with MEG at 96 hours |
Conclusion
The corrosion of stainless steels experienced a significant impact when exposed to NaCl combined with 4M MEG and oxygen in the presence of a CO2 atmosphere. This initial increase in corrosion rate was attributed to the undeviating attack of halide ions on the surface of the metal. With the passage of time (period of rotation), a decrease in corrosion rate was observed. The relationship between the period of rotation and corrosion rate followed a non-linear trend. The formation of a protective layer over the metal surface was held accountable for the decrease in corrosion rate.When submerged in corrosive medium, stainless steel exhibits active-passive behaviour which is due to the formation of either Cr2O3 or Molybdenum oxide on the metal surface and likely minimizes the corrosion action. The corrosion confrontation of stainless steel may be due to the passive film formation of chromium and molybdenum. SEM and EDX analyses of the samples revealed that the corrosion reaction predominantly occurred on irregular surfaces of the metal. This localized corrosion behavior further supported the observations made during the study. The research EDX light on the intricate interactions between different factors, such as solution composition, exposure time, and passive film formation that collectively influence the corrosion behaviour of stainless steels. Understanding these mechanisms is crucial for developing effective strategies to mitigate corrosion in practical applications.
Acknowledgements
The Canadian company CANMET Materials and Technology is gratefully acknowledged by the authors for providing the metals and helping with the SEM and EDX data recording.
References
- Kanimozhi, K.R.; Ph.D Thesis, 2014, Integrity of pipeline materials and Influence of Solvent Impurities in CCS Network – Injection and Storage., Avinashilingam University of Women, Coimbatore.
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T.; Energies, 2022, Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies.
CrossRef - Kazepidis, P.; Papadopoulos, A.I.; Tzirakis, F.; Seferlis, P.; Chem. Eng. Res. Des. 2021, 175, 209–222.Optimum design of industrial post-combustion CO2 capture processes using phase-change solvents.
CrossRef - Anusha, K.; Ph.D Thesis, 2010, Carbon Dioxide Capture by Chemical Absorption: A Solvent Comparison Study, Massachusetts Institute of Technology.
- Si Ali, B.; Ali, B.H.; Yusoff, R. and Aroua, M.K.; International Journal of Electrochemical Science,2012, 7,3835-3853,Carbon steel corrosion behaviors in carbonated aqueous mixtures of Monoethanolamine and 1-nbutyl-3-methlimidazolium tetrafluoroborate.
CrossRef - Davison, P.; Freund and Smith, A.; IEA Greenhouse Gas R&D Programme,2001, Pitting Carbon Black into the Ground.
- De Visser, E.; Hendriks, C.; and Barrio, M.; Molnvik, M. J.; Koeijer G.de.; Liljemark, S.; and Le Gallo Dynamis,Y.; International Journal of Greenhouse Gas Control, 2008, 2:4, 478-484,CO2 quality recommendations.
CrossRef - Ramgopal, T.; Francois, A.; and Sridhar, N.; CORROSION, 2009, paper no. 09256. Materials performance in supercritical CO2 environments.
- Papavinasam, S.; Jian Li,; Alex Doiron; Kourosh Zanganesh; Daryoush Emadi,; Carlos Salvador.; Ahmed Shafeen, and Allen Prett, CORROSION,2012, paper no.1270 Materials issues in CO2 capture, transport, and storage infrastructure.
- Kanimozhi, K.R.; Shyamala, R.; Papavinasam, S.; and Jian Li.; CORROSION,2014, Paper No.4074,Effect of Sodium Chloride concentration on the Corrosion of Carbon Steels and Stainless Steels in CO2 Environment at Atmospheric Pressure under Turbulent Flow Condition,.
- Kanimozhi, K.R.; Shyamala, R.; Papavinasam, S.; and Jian Li.; CORROSION, 2014, Paper No.4241, Effect of Monoethanolamine (MEA) on the Corrosion Rates of Carbon steels and Stainless Steels in CO2 Saturated NaCl Solutions.
- Mahdi Nouri, Jafar Mesbah, Soheil, B.; Shaibani, CORROSION, 2008, Paper no.08417,A verified computer model for corrosion rate prediction in a di-ethanol amine system using field data.
- Jon Kvarekval.; CORROSION, 2012, Paper no. 01554, Corrosion Layer Breakdown and Localized Corrosion in CO2/H2S Environments.
- Dillon, C. P.; 2001, Forms of Corrosion, MPE Engineers Inc.
- Melchers, R.E.; Corros. Sci., 2003, 45, 923–940, Mathematical modelling of the diffusion controlled phase in marine immersion corrosion of mild steel.
CrossRef - Melchers, R.E.; Jeffrey, R.; Corros. Sci., 2005, 47, 1678–1693, Early corrosion of mild steel in seawater.
CrossRef - Jr Callister, W.D.; 2003, Materials Science and Engineering an Introduction, Sixth Edition, John Wiley &Sons, lnc.
- Pineau, S.; Sabot, R.; Quillet, L.; Jeannin, M.; Caplat, C.H.; Dupont-Morral, I.; Refait, P.H. Corros. Sci. 2008, 50, 1099–1111, Formation of the Fe(II-III) hydroxysulphate green rust during marine corrosion of steel associated to molecular detection of dissimilatory sulphite-reductase.
CrossRef - Morcillo, M.; Chico, B.; de la Fuente, D.; Alcantara, J.; Odnevall Wallinder, I.; Leygraf, C. J. Electrochem. Soc., 2017, 164, C8–C16, On the Mechanism of Rust Exfoliation in Marine Environment.
CrossRef - Refait, P.; Grolleau, A.M.; Jeannin, M.; François, E.; Sabot, R. Corros. Sci, 2018, 130, 76–84, Corrosion of carbon steel at the mud zone/seawater interface: Mechanisms and kinetics.
CrossRef - Refait, P.; Grolleau, A.M.; Jeannin, M.; François, E.; Sabot, R. Corros. Sci. 2016, 111, 583–595, Localized corrosion of carbon steel in marine media: Galvanic coupling and heterogeneity of the corrosion product layer.
CrossRef - Kruger, J. Basics of Corrosion Science and Engineering, 2000, McGraw-Hill.
- Russell, M.; 2004 Chemical Surface Treatments on Stainless Steel, Public Relations Coordinator ASSDA.
- Washington, D. C.; United States Environmental Protection Agency, 2001, Report on the Corrosion of Certain Alloys.

This work is licensed under a Creative Commons Attribution 4.0 International License.










