Copper(II) Complexes as Functional Models for Lytic Polysaccharide Monooxygenase: A Kinetic Report
Balakrishnan Servaramuthu1,2 , Thavasilingam Nagendraraj1
, Thavasilingam Nagendraraj1 and Jamespandi Annaraj1*
and Jamespandi Annaraj1*
1Department of Materials Science, School of Chemistry, Madurai Kamaraj University, Madurai 625021, Tamil Nadu, India.
2Department of Chemistry, Vivekananda College, Thiruvedakam West, Madurai-625234, Tamil Nadu, India.
Corresponding Author E-mail: annaraj.chem@mkuniversity.org
DOI : http://dx.doi.org/10.13005/ojc/390232
Article Received on : 02 Jan 2023
Article Accepted on : 18 Mar 2023
Article Published : 31 Mar 2023
Reviewed by: Dr. Maher Khalid
Second Review by: Dr. Hemendra Bhandari
Final Approval by: Dr. Fasi Ulla
Mononuclear copper(II) complexes based on the symmetric tridentate N3-donor ligands bis(pyridin-2-yl)methyl)amine (HBPA, L1) and its methylated counterpart, di(2-pyridylmethyl)amine (MeDPA, L2) have been prepared, characterized, and demonstrated as the functional models for lytic polysaccharide monooxygenase. These complexes disrupt the synthetic substrate, such as p-nitrophenyl--D-glucopyranoside (-PNPG) into p-nitrophenol (PNP) and D-allose via oxidative cleavage as LPMOs do in nature. The observed spectroscopic and kinetic analysis have revealed that the reaction proceeds via copper(II) hydroperoxide as intermediate, whose electronic spectral signature has appeared at 350 nm in the electronic absorption spectra with the formation rate of 1.61 and 9.06 × 10-3 s1 respectively for complexes 1 and 2. Especially the obtained product was newly appeared at 400 nm, indicates the formation of p-nitrophenol with the rate of 7.52 and 5.45 × 10-3 s1.for complexes 1 and 2 respectively. These results affirm the ability of copper complexes as the functional models of LPMOs.
KEYWORDS:Biomimicking; Copper(II) complexes; Formation of D-Allose; LPMO models; Oxidative cleavage
Download this article as:| Copy the following to cite this article: Servaramuthu B, Nagendraraj T, Annaraj J. Copper(II) Complexes as Functional Models for Lytic Polysaccharide Monooxygenase: A Kinetic Report. Orient J Chem 2023;39(2). |
| Copy the following to cite this URL: Servaramuthu B, Nagendraraj T, Annaraj J. Copper(II) Complexes as Functional Models for Lytic Polysaccharide Monooxygenase: A Kinetic Report. Orient J Chem 2023;39(2). Available from: https://bit.ly/3m7Xn3l |
Introduction
Natural carbohydrate polymers which include starch, cellulose, and chitin provide a big renewable alternative to fossil fuels as a source of fuels and materials.1-3 Utilization of these polymers in large-scale industrial applications is still a difficult task due to their recalcitrant form for breaking into monomers.4-8 The enzymatic degradation of recalcitrant plant biomass has become very challenging in enzyme development for biomass utilization.9 A new enzyme called LPMOs are grouped in the enzyme families and termed Auxiliary Activities (AAs) in the Carbohydrate-Active Enzymes database (CAZy).10-13 Vaaje-Kolstad et al. uncover the capacity of LPMOs to degrade the native polymers into monomers.14 The LPMOs are more popular nowadays due to their ability to activate O2 to cleave the glycosidic bonds of polysaccharides.15-19 Vu et al have provided a detailed characterization of the active site of cupric ions in LPMOs.20 In addition, to employ O2 as an oxidant, Bissaro et al. demonstrated that the preferred co-substrate of LPMOs is H2O2 instead of using O2.21-23 Although several articles have stated that the polysaccharide has been selectively oxidized only at the C4 carbon, and both C1, as well as C4 through intermediates such as cupric-superoxide and cupric hydroperoxide species respectively, the nature of the intermediate involved in the C-H hydroxylation, is still unknown.24-27. Many functional models of copper complexes bearing N3 donor tridentate ligands have been demonstrated as catalysts for the degradation of b-PNPG to mimic the role of the native enzymes.28-32 Based on these backgrounds, we have prepared two mononuclear copper(II) complexes, bearing N3 donor tridentate ligands which are displaying LPMOs functions through the breakdown of b–PNPG into corresponding products such as PNP and D-allose.
Experimental
Reagents and Techniques
2-pyridinecarboxaldehyde, sodium borohydride, formaldehyde, 2-aminomethyl pyridine, copper(II) perchlorate hexahydrate and p-nitrophenyl-b-D-glucopyranoside were obtained from Alfa Aesar. Hydrogen peroxide (30%), hydrochloric acid, sodium carbonate, magnesium sulfate, and methanol were obtained from Merck. The ligands were confirmed by the nuclear magnetic resonance (NMR) spectroscopic technique using Bruker 400 MHz. The formation of complexes was confirmed by electrospray ionisation mass spectrometer (ESI-MS) (Agilent6530 LC/Q–TOF). Electronic absorption spectra recorded on the Agilent diode array spectrometer (Agilent 8453). Electrochemical measurements were performed using computer-controlled CH-Instruments, model 440. Electron paramagnetic resonance (EPR) spectral data were obtained from JEOL Model JES FA200. The degradation products were confirmed by gas chromatography mass spectrometer (GC-MS) Agilent 5977E.
Synthetic Procedures
Synthesis of N,N-bis(2-pyridylmethyl)amine (L1)
Both ligands HBPA (L1) and MeDPA (L2) were prepared by the early reported methods with slight modifications.33,34 2-pyridine carboxaldehyde (1.335 g, 12.5 mmol) was added to a solution containing 2-aminomethyl pyridine (1.35 g, 12.5 mmol) in MeOH (25 ml), the solution was undergoing colour change to dark brown. After 10 hrs sodium borohydride (0.945 g, 25 mmol) was slowly added, which turned the colour to a pale-yellow solution and the stirring was continued for another 3 h. Remove the all the volatiles under reduced pressure. Distilled water (25 ml) was added and neutralized the resulting aqueous solution with 32% hydrochloric acid, followed by extraction with dichloromethane. The combined organic extract was dried over anhydrous MgSO4 and put rotary evaporate to obtain the desired product, N,N-bis(2-pyridylmethyl)amine as yellow liquid. Yield, 0.6435 g (62.4%). 1H NMR (400MHz): CDCl3-d, d, 8.52 (d, 2H), 7.60 (t, 2H), 7.25 (d, 2H), 7.12 (t, 2H), 3.96 (s, 4H), 3.06 (s, 1H). 13C NMR: CDCl3-d, 159.66, 149.34, 136.57, 122.38, 122.04, 54.78 ppm
Synthesis of N-Methyl, N-bis(2-pyridylmethyl)amine (L2)
A reaction mixture containing bis[2-(2-pyridyl)methyl]amine (1.04 g, 5.225 mmol) and 1,2-dichloro-ethane (25 mL) was treated with 33% of aqueous formaldehyde (0.85 g, 10.45 mmol) under constant stirring. After 15 minutes, NaBH(OAc)3 (2.21 g, 10.45 mmol) was slowly added to the stirred solution and further stirred for 24 h at room temperature and quenched with the addition of an aqueous NaOH (2 M, 50 mL), the organic layer was separated and extracted with CH2Cl2 (3 × 50 mL portions). Combine the organic fractions, and dried over MgSO4, filtered and removed the solvent in vacuo. Taken up the obtained oily semisolid in diethyl ether (100 mL), filtered again and remove diethyl ether in vacuo to get the desired product as translucent golden coloured oil. Yield, 0.84 g (72.4%).1H NMR (400MHz): CDCl3, d, 2.31 (s, 3H), 3.77 (s, 4H), 7.15 (m, 2H), 7.52 (d, 2H), 7.66 (dt, 2H), 8.54 (d, 2H). 13C NMR CDCl3: 42.7, 63.6, 122.0, 123.1, 136.4, 149.0, 159.2 ppm.
Synthesis of copper(II) complexes
Copper(II) perchlorate in methanol was treated with the ligands (L1 or L2) in dichloromethane and the blue colour solids were separated and dried (Scheme 1).
 |
Scheme 1: Synthesis of copper(II) complexes of L1 and L2 ligands (a) and structures model of LPMOs (b). |
Results and Discussion
Synthesis and Characterization
[Cu(L1)(H2O)2](ClO4)2 (1)
Cu(ClO4)2.6H2O (0.37 g, 1 mmol) in 4 mL of methanol was drop-wisely added to a stirring solution of ligand, L1 (0.200 g,1 mmol) in 4 mL of dichloromethane (DCM), this reaction mixture was stirred for 2 h at room temperature. The obtained blue colour solid product was precipitated, filtered, and dried for further purposes.
[Cu(L2)(H2O)2] (ClO4)2 (2)
The complex 2, the above same procedure has been adopted using ligand L2 instead of L1.
These prepared complexes were used as functional models of LPMOs. The obtained blue colour crystalline solids were confirmed by ESI-MS in methanol, where the peak appears at m/z 297.0090 for [C12H16CuN3O2]+ and m/z 307.0377 for [C12H14CuN3 + 2Na] for complex 1 and ESI-MS: m/z 360.1803 for [C13H19CuN3O2 + 2Na] for complex 2 respectively (Fig. 1).
 |
Figure 1: ESI-MS spectra of complex 1 (a) and 2 (b) in methanol solution. |
Electronic Spectra, Redox Properties, and EPR Studies
Both these complexes, 1 and 2 have shown intense and broad absorptions at 645 nm in water (Fig.2a and Table 1) which corresponds to d-d transitions, interestingly they were closely related to the absorption of previously reported LPMO model (655 nm) complexes.35 Their well-defined Cu(II)/Cu(I) redox potentials (-0.18 and -0.19 V vs normal hydrogen electrode (NHE) respectively for complexes 1 and 2 (Fig.2b Table1) have been observed in aqueous carbonate buffer, which are found to be lower than the previously reported enzymatic systems.36-38. Furthermore, their square pyramidal geometries and oxidation states were confirmed by EPR spectra (Fig.2c) dimethylforamide (DMF) (8:2) at 70 K in methanol and the obtained axial EPR parameters (g|| =2.2572, A|| =158 × 10-4 cm-1 and g⊥=2.0204) for complex 1 and (g|| =2.2604, A|| =179 × 10-4 cm-1 and g⊥=2.0221) for complex 2 were in good agreement with those obtained for LPMOs.39-41
 |
Figure 2: Electronic spectra (a), cyclic voltammograms (b) and EPR (c) of complexes 1 and 2 (1 × 10-3 M) in the presence of carbonate buffer at 25oC. The scan rate 50 mV s-1.. |
Table 1: Electronic spectral and redox data for complexes 1 and 2 in carbonate buffer at pH 10.
|
Complex |
Electronic spectra λmax nm, (εmax M-1 cm-1) |
Redox data |
||||
|
Epc(V) |
Epa(V) |
ΔE (V) |
E 1/2(V) |
E 1/2(V) vs NHE |
||
|
1 |
645 (432.9) |
-0.46 |
0.-31 |
-0.15 |
-0.39 |
-0.18 |
|
2 |
645 (435.1) |
-0.45 |
-0.33 |
-0.12 |
-0.39 |
-0.19 |
Formation of Cu-OOH species
The formation of copper(II) hydroperoxide species in both complexes, 1 and 2 were obtained by the addition of H2O2 solution in carbonate buffer at pH 10 (Fig. 3), which appeared around 350 nm in the electronic absorption spectra. The complex containing water molecules, which was deprotonated at basic medium using carbonate buffer at pH 10 to form Cu(II) hydroxyl, which was treated with hydrogen peroxide to form copper hydroperoxide (CuII-OOH) species.
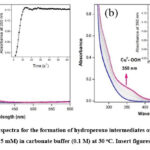 |
Figure 3: The absorption spectra for the formation of hydroperoxo intermediates of complexes 1 (a) and 2 (b) (0.05 mM) with H2O2 (0.5 mM) in carbonate buffer (0.1 M) at 30 oC. Insert figures: Plot of 1+Abs. vs Time. |
Oxidation cleavage of β-PNPG
The oxidative cleavage of β-PNPG was examined by UV-visible spectroscopic technique. as well as GC-MS. The oxidized products such as PNPand D-Allose were monitored in different temperatures with varying concentrations of catalyst, substrate, and oxidant (H2O2) at 400 nm (Scheme 2).
 |
Scheme 2: Oxidative cleavage of β-PNPG and hydrogen peroxide with catalyst. |
Kinetic reactions were conducted in the presence of β-PNPG as well as aqueous H2O2 with complexes 1 and 2 for a ratio of 1:10:10 mixture in carbonate buffer at pH 10 to confirm the LPMO-like reactivity of the complexes. Thus, the kinetic spectral results lead to observing an absorption band at 400 nm that may correspond to PNP (Fig.4,5). In addition, the significant shift in the electronic spectra indicates that the oxidative cleavage reaction will be initiated when the substrate is combined with both complexes, either 1 or 2 in the presence of hydrogen peroxide (Fig.6). The reaction did not go as planned if either complex or hydrogen peroxide were missing. The final catalytic solution consisting of a catalytic amount of the model complex 1 or 2 (20 mmol), the model substrate (200 mmol), with 30% aqueous hydrogen peroxide (200 mmol) in carbonate buffer (2.0 mL) were passed over a silica column after 2 h, in order to confirm the product analysis (PNP and D-allose) by GC-MS (Fig. 7). A blank reaction was also carried out under the same conditions, which did not yield any product as we anticipated.
 |
Figure 4: The electronic spectra for the kinetic reaction of complexes 1 (a) and 2 (b) (0.05 mM) |
 |
Figure 5: (a) Time dependant absorption spectra for the kinetic reaction of complexes 1 and 2 (0.05 mM) with the substrate (p-nitrophenyl-β-D-glucopyranoside, 0.5 mM) |
 |
Figure 6: The absorption spectra for the reaction between complexes 1 or 2 (0.05 mM) |
 |
Figure 6: The absorption spectra for the reaction between complexes 1 or 2 (0.05 mM) |
Conclusion
A simple mononuclear copper(II) complexes were synthesized, characterized, and demonstrated as functional models for lytic polysaccharide monooxygenase. Their geometrical and physicochemical properties were similar to those of the active sites of LPMOs. The catalytic activity of the copper(II) complexes were successfully evaluated using the polysaccharide model substrate. This model catalytic reaction proceeded via copper(II) hydroperoxide as an intermediate which is responsible for the degradation of the b-PNPG leading to the formation of oxidized products such as PNP and D-allose via oxidative cleavage. The formation of p-nitrophenol declares the ability of copper complexes to mimic the biological processes of LPMOs. This makes the present complexes excellent models which illustrate both the structural properties and reactivity of the active sites of LPMOs. In order to assess the contributions of N-H structural alterations and quantification products derived from the model complex, more research is being done.
Acknowledgment
The authors acknowledge the University Grant Commission (UGC-SERO, File no. TNMK006), Hyderabad and RUSA MKU (File No. 002/RUSA/MKU/2020-2021) for the financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meier, K. K.; Jones, S. M.; Kaper, T.; Hansson, H.; Koetsier, M.J.; Karkehabadi, S.; Solomon, E. I.; Sandgren, M.; Kelemen, B. Chem. Rev., 2018, 118, 2593−2635.
CrossRef - Hemsworth, G.R.; Johnston, E.M.; Davies, G.J.; Walton, P.H. Trends Biotechnol., 2015. 33, 747–761.
CrossRef - Quinlan, R. J.; Sweeney, M. D.; Lo Leggio, L.; Otten, H.; Poulsen, J. C. N.; Johansen, K. S.; Krogh, K. B. R. M.; Jorgensen, C. I.; Tovborg, M.; Anthonsen, A.; Tryfona, T.; Walter, C. P.; Dupree, P.;Xu, F.; Davies, G. J.; Walton, P. H. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 15079−15084.
CrossRef - Beeson, W. T.; Phillips, C. M.; Cate, J. H. D.; Marletta, M. A. J. Am. Chem. Soc., 2012, 134, 890−892.
CrossRef - Hemsworth, G. R.; Davies, G. J.; Walton, P. H. Curr. Opin. Struct. Biol., 2013, 23, 660−668.
CrossRef - Beeson, W. T.; Vu, V. V.; Span, E. A.; Phillips, C. M.; Marletta, M. A. Annu. Rev. Biochem., 2015, 84, 923−946.
CrossRef - Chylenski, P.; Bissaro, B.; Sorlie, M.; Rohr, A. K.; Varnai, A.; Horn, S. J.; Eijsink, V. G. H. ACS Catal., 2019, 9, 4970−4991.
CrossRef - Forsberg, Z.; Sorlie, M.; Petrovic, D.; Courtade, G.; Aachmann,F. L.; Vaaje-Kolstad, G.; Bissaro, B.; Rohr, A. K.; Eijsink, V. G. H..Curr. Opin. Struct. Biol., 2019, 59, 54−64.
CrossRef - Sandra T. M .; Joel Cherry. Adv Biochem Engin/Biotechnol., 2007, 108, 95–120.
- Solomon, E. I.; Heppner, D. E.; Johnston, E. M.; Ginsbach, J. W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M. T.; Kjaergaard, C. H.; Hadt, R. G.; Tian, L. Chem. Rev., 2014, 114 (7), 3659−3853.
CrossRef - Hemsworth, G. R.; Henrissat, B.; Davies, G. J.; Walton, P. H. Nat. Chem. Biol., 2014, 10, 122-126.
CrossRef - Cantarel, B. L.; Coutinho, P. M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. Nucleic Acids Res., 2009, 37, D233−D238.
CrossRef - Frandsen, K.E.H.; Lo Leggio, L. IUCrJ., 2016, 3, 448–467.
CrossRef - Vaaje-kolstad, G.; Westereng, B.; Horn, S. J.; Liu, Z.; Zhai, H.;Sorlie, M.; Eijsink, V. G. H. Science. 2010, 330, 219−223.
CrossRef - Walton, P. H.; Davies, G. J. Curr. Opin. Chem. Biol., 2016, 31, 195−207.
CrossRef - Lo Leggio, L.; Simmons, T. J.; Poulsen, J.-C. N.; Frandsen, K. E. H.; Hemsworth, G. R.; Stringer, M. A.; von Freiesleben, P.; Tovborg, M.; Johansen, K. S.; De Maria, L.; Harris, P. V.; Soong, C.-L.; Dupree, P.; Tryfona, T.; Lenfant, N.; Henrissat, B.; Davies, G. J.; Walton, P. H. Nat. Commun., 2015, 6, 5961−5969.
CrossRef - Vu, V. V.; Beeson, W. T.; Span, E. A.; Farquhar, E. R.; Marletta, M. A..Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 13822−13827.
CrossRef - Lo Leggio, L.; Simmons, T. J.; Poulsen, J. C. N.; Frandsen, K. E.H.; Hemsworth, G. R.; Stringer, M. A.; von Freiesleben, P.; Tovborg, M.; Johansen, K. S.; De Maria, L.; Harris, P. V.; Soong, C. L.; Dupree, P.; Tryfona, T.; Lenfant, N.; Henrissat, B.; Davies, G. J.; Walton, P. H.. Nat. Commun., 2015, 6, 5961.
CrossRef - Simmons, T. J.; Frandsen, K. E. H.; Ciano, L.; Tryfona, T.;Lenfant, N.; Poulsen, J. C.; Wilson, L. F. L.; Tandrup, T.; Tovborg,M.; Schnorr, K.; Johansen, K. S.; Henrissat, B.; Walton, P. H.; LoLeggio, L.; Dupree, P. Nat. Commun., 2017, 8, 1064
CrossRef - Vu, V. V.; Ngo, S. T. Coord. Chem. Rev. 2018, 368, 134–157.
CrossRef - Bissaro, B.; Rohr, A. K.; Muller, G.; Chylenski, P.; Skaugen, M.;Forsberg, Z.; Horn, S. J.; Vaaje-Kolstad, G.; Eijsink, V. G. H. Nat. Chem. Biol., 2017, 13, 1123−1128.
CrossRef - Filandr, F.; Man, P.; Halada, P.; Chang, H. H.; Ludwig, R.;Kracher, D. Biotechnol. Biofuels, 2020, 13, 37.
CrossRef - Hangasky, J. A.; Iavarone, A. T.; Marletta, M. A. Proc. Natl.Acad. Sci. U. S. A. 2018, 115, 4915−4920.
CrossRef - Hedegard Dhar, D.; Tolman, W. B. J. Biol. Inorg. Chem., 2017, 22, 1−9.
CrossRef - Neira, A. C.; Martínez-Alanis, P. R.; Aullón, G.; Flores-Alamo, M.; Zerón, P.; Company, A.; Chen, J.; Kasper, J. B.; Browne, W. R.;Nordlander, E. ACS Omega, 2019, 4 (6), 10729−10740.
CrossRef - Kim, S.; Stahlberg, J.; Sandgren, M.; Paton, R. S.; Beckham, G.T. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (1), 149−154.
CrossRef - Elwell, C. E.; Gagnon, N. L.; Neisen, B. D.; Dhar, D.; Spaeth, A.D.; Yee, G. M.; Tolman, W. B. Chem. Rev., 2017, 117 (3), 2059−2107.
CrossRef - Concia, A. L.; Beccia, M. R.; Orio, M. ; Ferre, F. T.; Scarpellini, M.; Biaso, F.; Guigliarelli, B.; Reglier M.; Simaan, A. J. Inorg. Chem., 2017, 56, 1023-1026.
CrossRef - Muthuramalingam, S.; Maheshwaran, D.; Velusamy, M.; Mayilmurugan, R. J. Catal., 2019, 372, 352-361.
CrossRef - Fukatsu, A.; Morimoto, Y.; Sugimoto, H.; Itoh, S. Chem. Commun., 2020, 56, 5123-5126.
CrossRef - Gagnon, N.; Tolman, W.B. Acc Chem. Res., 2015, 48, 2126–2131.
CrossRef - Itoh, S. Acc. Chem. Res., 2015, 48 2066–2074.
CrossRef - Liu, Y.; Xiang, R.; Du, X.; Ding, Y.; Ma, B. Chem. Comm., 2014, 50, 12779-12782.
CrossRef - Budweg, S.; Junge, K.; Beller, M. Chem. Comm., 2019, 55, 4143-14146.
CrossRef - Solomon, E.I., Proc. Natl. Acad. Sci. U.S.A. 2014, 111 8797–8802.
CrossRef - Hemsworth, G.R.; Johnston, E.M.; Davies, G.J.; Walton, P.H., Trends Biotechnol., 2015, 33, 747–761.
CrossRef - Beeson, W.T. ;Vu V.V. ; Span, E.A.; Phillips, C.M.; Marletta M.A. Annu. Rev.Biochem., 2015, 84, 923–946.
CrossRef - Garajova, S.; Mathieu, Y.; Beccia, M.R.; Bennati-Granier, C.; Biaso, F;. Fanuel, M.; Ropartz, D.; Guigliarelli, B.; Record, E;. Rogniaux, H.; Henrissat, B.; Berrin, J.G. Sci. Rep., 2016, 6, 28276.
CrossRef - Frandsen, K.E.H.; Simmons, T.J.; Dupree, P.; Poulsen, J.-C.N.; Hemsworth, G.R.; Ciano, L.E.M.; Johnston, M.; Tovborg, K.S.; Johansen, P.; von Freiesleben, L.; Marmuse, S.; Fort, S.; Cottaz, H.; Driguez, B.; Henrissat, N.; Lenfant, F.; Tuna, A. Baldansuren,; G.J. Davies, L.; Lo Leggio,; P.H. Walton. Nat. Chem.Biol., 2016, 12, 298–303.
CrossRef - Halfen,, J.A.; Uhan J.M,; Fox, D.C.; Mehn, M.P;. Que, L. Inorg. Chem., 2000, 39 4913–4920.
CrossRef - Zhu, Q; Lian, Y; Thyagarajan, S;. Rokita, S.E; Karlin. K.D;., Blough; N.V. J. Am. Chem. Soc., 2008, 130, 6304–6305.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









