Antimicrobial Activity of Calix[4]Pyrrole-entrenched Silver Nanoparticles and its Application as Colorimetric and Spectrophotometric Sensing of L-Histidine
1Department of Chemistry, Faculty of Science, Ganpat University, Mehsana, Gujarat, India.
2Department of Chemistry, Tolani College of Arts and Science, KSKV Kutch University, Bhuj, Gujarat, India
Corresponding Author E-mail: drkdbhatt@outlook.com
DOI : http://dx.doi.org/10.13005/ojc/390104
Article Received on : 07 Dec 2022
Article Accepted on : 09 Jan 2023
Article Published : 09 Jan 2023
Reviewed by: Dr. Nabil Alhemiary
Second Review by: dralhemiary@gmail.com
Final Approval by: Dr. Rohit Madhukar Nikam
A calix[4]pyrrole tetrahydrazide functionalized silver nanoparticles (CPTPH-AgNPs) coupled with colorimetric findings were prepared. In the variety of essential amino acids, it detected the L-Histidine exclusively with an LOD of 6.1 μM and LOQ of 18.5 μM. The CPTPH-AgNPs were exhibited the SPR spectrum at 408 nm, which further characterized with TEM, EDEX and SAED in that they show the monodispersed spherical morphology of AgNPs with 13 ± 2 nm size. The antimicrobial potential of CPTPH-AgNPs towards Gram positive bacterial - Staphylococcus aureus, Bacillus subtilis, Gram negative bacteria - Escherichia coli, Pseudomonas aeruginosa and fungi - Aspergillus niger was evaluated.; in which it is found to give reasonable response comparable to standard antibiotic drugs.
KEYWORDS:Calix[4]pyrrole; Metal Nanoparticles; Silver Nanoparticles; Supramolecular Chemistry
Download this article as:| Copy the following to cite this article: Pomal N. C, Bhatt K. D, Kundariya D. S. Antimicrobial Activity of Calix[4]Pyrrole-entrenched Silver Nanoparticles and its Application as Colorimetric and Spectrophotometric Sensing of L-Histidine. Orient J Chem 2023;39(1). |
| Copy the following to cite this URL: Pomal N. C, Bhatt K. D, Kundariya D. S. Antimicrobial Activity of Calix[4]Pyrrole-entrenched Silver Nanoparticles and its Application as Colorimetric and Spectrophotometric Sensing of L-Histidine. Orient J Chem 2023;39(1). Available from: https://bit.ly/3CyRyB7 |
Introduction
Because of their wide applicability in biology and therapeutics, the design, production, and functionalization of metal nanoparticles have piqued the interest of many researchers in recent years. Especially, gold nanoparticles (AuNPs) and silver nanoparticles (AgNPs) are highly efficient materials for the last few decades for their physical, optical and size-relevant properties1. To achieve nano-sized entities; various approaches are been utilized among which chemical reduction is a widely employed method, utilizing various reducing agents and surfactants which impart stability to NPs and offer separation which disables the acts of aggregation between them2 is the current interest of researchers worldwide. The major challenge before the nano-chemists are to make very stable and tunable-sized nanoparticles with expedient properties of employability.
The combination that fulfils the above criteria are metal nanoparticles encapsulated using the calix[4]pyrrole which created the excited and emerging new class of compounds in the last decade3. Calix[4]pyrrole functionalized metal nanoparticles had sought the attention of chemists due to its inherent void and web-like structure4, equipping it for various roles, notably in the field of nanotechnology providing the two-fold application of reductant and stabilizing agent for metal ions which opens the doors of numerous applications ranging from catalysis5 to sensing6.
Sensing the different small molecules, anions, and cations7 is important for their significant contribution to the environment, living organisms and industries8. From the catalogue of small molecules, amino acids play a vital role in the human body and are the main building blocks of numerous proteins9. In the sub-class of amino acids, the essential amino acids are those which are not generated endogenously in the human body, are Threonine, Tryptophan, Phenylalanine, Valine, Lysine, Histidine, Leucine, Methionine and Isoleucine10. In this index, the Histidine (or L-Histidine) is having unique roles in the human body and its metabolisms including, as a pH buffer due to its imidazole ring (pKa varies from 6.0 to 7.1)11, metal ion chelation with some cations, especially with an iron of haemoglobin12, it demonstrates the antioxidant activity by scavenging the reactive nitrogen and reactive oxygen entities13, producing histamine in the body14 other than that L-histidine is having roles in industries as an antioxidant for preserving milk powder and added to feed of cow cereals and supplements15 and pharma industries producing a nutritious product from it16. WHO has suggested that 10 mg/kg per day L-Histidine in the diet of adults17 justifies the theoretical rationale of its recommendations based on its distinct chemical characteristics and physiological roles18. Chronic renal disease can be linked to low L-Histidine levels, which contributes to L-Histidine metabolism instability and irregularities in the proportions of essential byproducts including histamine19 on the other hand high concentration of L-Histidine leads to poor memory, depression, weakness, headache and nausea, affecting the tastes and smell severely20.
Hence, the detection of L-Histidine is very crucial by means of biology, clinical chemistry and industrial perspective. Significant work has been put into incipient techniques for detecting L-Histidine including; fluorescent probes, electrochemical sensing, mass spectrometry, atomic spectrometry, electrophoresis, voltammetry, amperometry, chromatography and resonance light scattering21-22. Although, these methods have produced extremely precise and focused findings, at the same time they are cumbersome, laborious, demanding sophisticated instrument as well as requires expensive solvents, reagents and desired conditions, more volume of samples with large lab space. Thus, the above constraints and barriers have led the researchers to design and develop new colorimetric, naked-eyes detectors with micro-concentrations of samples and on-sight results.
In the present study, very stable silver nanoparticles (CPTPH-AgNPs) are synthesised using the calix[4]pyrrole tetrapropanehydrazide as a reducing and stabilizing agent which acts as a nano-sensor selectively and sensitively for L-Histidine amongst other targeted amino acids by colorimetry and spectrophotometry. Moreover, it exhibited anti-microbial activity.
Experimental
Chemical and Reagents
All the amino acids, levulinic acid, boron trifluoride ethyl etherate, hydrazine hydrate and silver nitrate were purchased from Sigma-Aldrich. The other solvents, reagents and chemicals were analytical reagent grade obtained from a local chemical supplier and used without further purification. REMI-2 MLH Magnetic stirrer was used for the synthesis steps. TLC plates were fluorescent active (F-254) provided by Merck. Micropipette (100 – 1000 μL) was obtained by Microlit (Lucknow, India). All the glass wares used in experiments were precisely calibrated and washed two times with double distilled water.
Instruments
A 97 VEEGO equipment was used to determine uncorrected melting points (VEEGO, Mumbai, India). Bruker FT-IR ALPHA-II was used to calculate each compound’s infrared spectra (expressed in cm-1). The SHIMADZU UV-1900 device was used for UV-Visible studies. version 18.0.0.231 (4029) version of ChemDraw Professional software was used to construct the chemical names and chemical structures of compounds. To examine the morphology of the fabricated AgNPs, the JEOL model JEM-2100 TEM machine was used to generate images. Proton (1H) and Carbon (13C) NMR spectra were obtained employing DMSO as a solvent on Brucker ASCENDTM 400 instrument. The mass spectrum was measured on SHIMADZU Nexera 2020.
Synthetic Procedures of Compounds and Silver Nanoparticles
Synthesis of calix[4]pyrrole tetrapropionic acid (CPTPA)
The carboxylic acid functionalized calix[4]pyrrole (CPTPA) was synthesized according to slight modification in a previously reported procedure23 using BF3·OEt2 acid-catalyzed condensation of pyrrole with levulinic acid. The modified detailed procedure is, the addition of 1.16 mL (10 mmol) levulinic acid and 0.67 mL (10 mmol) of freshly distilled pyrrole to 30 mL of methanol was made completed dropwise. After stirring in an ice bath for two hours in a nitrogen environment, BF3·OEt2 was dropwise made to add to the aforesaid mixture for 15 minutes and left to reflux overnight. After completion of the reaction, the reaction mixture was quenched with ice-cold water (3 × 25 mL). To this triethylamine was added to remove any unreacted acid. The dark brown precipitations were then quickly filtered and made soluble to diethyl ether and passed through the Na2SO4 bed to make it moisture-free and then isolated using hexane (3 × 10 mL) to get free-flowing powder. This dark brown powder is purified using column chromatography, which obtained the off-white solution allowed for concentration under a vacuum which eventually gave off-white powder (Scheme-1).
Off-white Solid (78% yield), M.P.205ºc, FT-IR (KBr disk):3273, 2936, 1714, 1475, 1163, 1041 in 1/cm. 1H NMR (400-MHz, DMSO): δ = 1.48 (s, 12H, CH3), 1.92 (t, 8H, CH2), 2.13 (t, 8H, CH2), 5.73 (d, 8H, C-H, ArH); 10.17 (m, 4H, Pyrrolic NH), 11.94 (s, 4H, COOH). 13C NMR (400 MHz, DMSO): δ = 175.59, 139.48, 105.30, 42.49, 29.74, 28.90, 24.12. ESI-MS m/z: 661.00 (M + 1) + for Chemical Formula: C36H44N4O8.
Synthesis of calix[4]pyrrole tetrapropanehydrazide (CPTPH)
The CPTPH was previously reported utilizing ester functionalized calix[4]pyrrole (CPTM)24-25. Here, the same supramolecular species was synthesised using a modified approach from acid functionalized calix[4]pyrrole. The comprehensive updated process is described as; the 0.20 g (0.30 mmol) CPTPA was made soluble in ethanol and 1.25 mL (25 mmol) hydrazine hydrate was added dropwise for 20 minutes and stirred under nitrogen atmosphere for 1 hour. After that, The mixture vortexed for 48 hours. The synthesis progress was inspected periodically, using thin layer chromatography. To obtain the crude product from the reaction mixture, it was quenched with frigid water. On quenched, a powdery substance appeared and dispersed in the mixture, that was centrifuged at 5000 RPM three times, resulting in the settlement of fine powder at the bottom of the tube which was collected and washed with de-ionized water (3 × 25 mL) and recrystallized in hot methanol gave pale yellow powder. This powder was isolated by employing n-hexane (3 × 10 mL). This CPTPH (Scheme-1) was then purified using column chromatography.
White Solid (60% yield), M.P.202ºc, FT-IR (KBr disk):3277, 2968, 1610, 1267, 1166, 1039 in 1/cm. 1H NMR (400 MHz, DMSO): δ = 1.74 (s, 12H, CH3), 2.09 (t, 8H, CH2), 2.27 (t, 8H, CH2), 4.10 (s, 8H, NH2) 5.73 (d, 8H, C-H, ArH); 8.89 (t, 4H, NH), 9.36 (s, 4H, Pyrrolic NH). 13C NMR (400 MHz, DMSO-d6): δ = 169.90, 141.92, 107.30, 43.50, 32.53, 31.20, 25.90. ESI-MS m/z: 717.42 (M+1)+ for C36H52N12O4.
 |
Scheme 1: Synthesis of CP Acid and CP Hydrazide |
Synthesis of calix[4]pyrrole tetrapropanehydrazide functionalized silver nanoparticles (CPTPH-AgNPs)
A highly stable AgNPs were synthesised in a one-pot procedure, employing tetrapropanehydrazide calix[4]pyrrole holding four -NH-NH2 arms and used as a reducing and capping agent. All the glass wares were carefully scrubbed with freshly prepared aqua-regia and afterwards rinsed with de-ionized water before drying for two hours in a 105˚ c oven. In the usual protocol, 50 mL of 1.0 mM silver nitrate was brought to boiling. Drop-wise addition of 10 mL of CPTPH ligand was accomplished to the boiling AgNO3 solution and stirred vigorously; within 15 minutes, colour of the solution turned yellow to colourless, signifying the generation CPTPH capped AgNPs. Following this, the solution was stirred continuously at 600 RPM for 4 hours at ambient temperature. The solution was then stored at 4˚c for further experiments.
Upon successfully preparing CPTPH-AgNPs, the SPR spectrum was recorded on a UV-Visible spectrophotometer, with a characteristics peak at 408 nm (Fig.1) signified the spherical shape of silver nanoparticles.
 |
Figure 1: UV-Visible spectrum of CPTPH-AgNPs (SPR peak). |
Stock solution preparation for spectrophotometry
Essential amino acids were chosen for the colorimetric and spectrophotometry assay in the present study. The stock solution of CPTPH-AgNPs (0.0053%) and amino acids (1.0 μmol/L) i.e. Threonine, Tryptophan, Phenylalanine, Valine, L-Lysine, L-Histidine, L-Leucine, Methionine and Isoleucine were prepared in methanol, from the stock solutions, 2.5 mL of each amino acid solution mixed with 2.5 mL of CPTPH-AgNPs, enables the effective concentration of 0.5 μmol/L. After proper mixing, each glass tube was taken for the spectrophotometric assays.
Optimization study of CPTPH-AgNPs
The aggregation of CPTPH-AgNPs was assessed by varying the pH (4.0 to 10.0) utilizing 0.1 M HCl and 0.1 M NaOH. CPTPH-AgNPs were found to be stable at pH 7.0 (Fig.2 (a)), but aside from that, they tended to aggregate after a few hours even when they were sonicated. The CPTPH-AgNPs were proved to be stable for 30, 60, 90 and 120 days with minute modification in the SPR band (Fig.2 (b)). The CPTPH-AgNPs were investigated for temperatures ranging from 10˚ to 50˚c, the SPR peak was intact from 10˚ to 30˚c and slight deviation occurred for 40˚ and 50˚c (Fig.2 (c)). From these analytical studies, it was inferred that CPTPH-AgNPs were found stable at pH 7.0 and 10˚ to 30˚c. Hence, all other interrogations were conducted using the same criteria of pH and temperatures.
 |
Figure 2: Optimization study of CPTPH-AgNPs: (a) pH vs Wavelength, (b) Days vs Wavelength, (c) Temperature vs Wavelength |
Antimicrobial action of CPTPH-AgNPs
The calix[4]pyrrole tetrahydrazide functionalized silver nanoparticles (CPTPH-AgNPs) were studied as an antimicrobial agent against various micro-organisms cultures (Table-1).
Table 1: Cultures used in the Antimicrobial assay.
|
Culture Name |
Strain Name |
Reference of Strain |
|
Gram positive bacteria |
Staphylococcus Aureus |
MTCC1430/ATCC12600 |
|
Bacillus Subtilis |
MTCC121/ATCC6051 |
|
|
Gram negative bacteria |
Escherichia coli |
MTCC448/ATCC9637 |
|
Pseudomonas Aeruginosa |
MTCC1934/ATCC10415 |
|
|
Fungi |
Aspergillus niger |
MTCC514/ATCC10581 |
|
MTCC: The Microbial Type Culture Collection and Gene Bank (MTCC), CSIR-Institute of Microbial Technology, Sector 39-A, Chandigarh – 160036, INDIA. |
||
Method
The disc diffusion method i.e. Agar diffusion study was used for the antimicrobial action having a disc size of 6 mm26-27.
Concentration of compounds
DMSO as a solvent was utilized to prepare the stock solution of each compound. The amount for the study was determined in increments of 25, 50, 75, and 100% per disc. As a standard, Hi-media antibacterial drugs, chloramphenicol (10 μg/disk) and amphotericin-B (100 units/disk) were humidified using the optimal volume of water.
Media for microbial assay
Nutrient agar (Hi-media) in g·L-1 was the microbial medium being used for Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, and Escherichia coli. Sodium chloride, 5, beef extract 10, peptone 10 (all solutions in g·L-1) at pH 7.2. Potato dextrose agar was used as a microbiological medium for Aspergillus Niger fungus (all ingredients of Hi- media) with composition; (in g·L-1) potatoes infusion 200, Dextrose 20, Agar 15 and pH 5.6 ± 0.2 (at 25°C).
Characterization of CPTPH-AgNPs
For various kinds of applications and studies, the different types of AgNPs with distinct sizes and shapes are synthesized. Nowadays, the design of nanoparticles is done in such a way that it satisfies the needs of applications successfully and completely. So, in the current research, the CPTPH-AgNPs were prepared with aim of sensing of analyte and its activities against microbes, which requires a spherical shape of AgNPs with SPR should have appeared between the range of 400 to 500 nm in UV-Visible spectroscopy. The result achieved was in good agreement with according to aim, the SPR spectra appeared at 408 nm, and the colour of the nano-colloid solution was yellow (Fig.2). Transmission Electron Microscopy (TEM) results (Fig.3 (a), (b)) have confirmed the nano-silver of size 13 ± 2 nm, monodispersed, and possessing the spherical shape. SAED, i.e. Selected Area Electron Diffraction pattern (Fig.3 (c)) shows bright spots which imply to crystalline nature of CPTAH-AgNPs. The Energy Dispersive X-ray (EDX) findings (Fig.4) asserted the silver element is present in the sample of colloid solution. All the above data corroborated that the yellow colloidal solution embraced the silver nanoparticles with a size around 13 to 15 nm, SPR spectra at 408 nm and crystalline elemental silver in it.
 |
Figure 3: (A, B) TEM images at 5 nm and 20 nm scale, (C) SAED, and (D) Particle size distribution of CPTPH-AgNPs |
 |
Figure 4: Energy dispersive X-ray of CPTPH-AgNPs |
Result and Discussion
Mechanism of formation of CPTPH-AgNPs
The interaction chemistry at the nano-level is still in the development stage and researchers are putting their best efforts to discover the whole process without error. Although, some past computational studies over this point can be endorsed and can be reached to the conclusion on the mechanism of formation nanoparticles by the account of; (i) The formation of silver nanoparticles can be explained by the fact that when CPTPH is applied as a reducing and stabilizing agent, the carbonyl oxygen and eight nitrogen of hydrazide are accessible to give electrons to the silver ions (Ag+) of AgNO328. (ii) The stabilizing behaviour of CPTPH can be attributed to the intermolecular hydrogen bonding between ligand and metal atoms due to the proximity of ligand and its intactness over the surface of metal atoms which restrict the conformational flexibility of ligand29. (iii) The ligand-to-metal charge transfer plays a very crucial role in the formation of AgNPs30. Apart from the above markings, the size, shape, area, electronic structure as well as the local electrolytic environment affect the capping actions of ligands and formation of nanoparticles and their stability31. Thus, the point to draw from the above discussions is that the successful formation of stable CPTPH-AgNPs is the result of non-covalent interactions.
Colorimetry Assay of L-Histidine
2.5 mL (1 μmol/L) solution of each amino acid; i.e. Threonine, Tryptophan, Phenylalanine, Valine, L-Lysine, L-Histidine, L-Leucine, Methionine and Isoleucine were mixed separately with 2.5 mL of CPTPH-AgNPs in different glass tubes. For 10 minutes, all solution blends in glass tubes were incubated at room temperature, to observe any visual change in the colour. After 10 minutes, except L-Histidine, all other solutions were found to have the same yellow colour of CPTAH-AgNPs colloid (Fig.5). The glass tube of CPTPH-AgNPs + L-Histidine exhibited colourless solution as seen in the 8th bottle in Fig.5. The transformation of colour from yellow to colourless of metal nanoparticles in the visual assay can be associated either with aggregation or reduction in the size of metal nanoparticles32. It is reported that when analytes have greater interactions with metal nanoparticles it pulls away the capping ligand from the surface of nanoparticles and leaves behind the bare nanoparticles in the solution33 which causes the shift in the SPR spectra. The concentration of analytes also affects the capping actions on the surface of metal nanoparticles34. Evidently, in the spectrophotometric titrations, it was observed that with high concentrations of L-Histidine colour disappear (Fig.6) and that was observed in SPR spectra too.
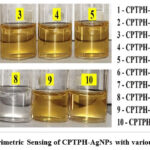 |
Figure 5: Colorimetric Sensing of CPTPH-AgNPs with various amino acids |
 |
Figure 6: Colorimetric results of CPTPH-AgNPs on addition of different amounts of L-Histidine |
Spectrophotometric Study
UV-Visible study of different Amino Acids with CPTPH-AgNPs
Various amino acids were taken for spectrophotometric evaluation, in the whole class of this study, only L-Histidine was turned out to be demonstrated the peculiar peak (Fig.7) along with colorimetric outcome from yellow to a colourless solution. The experimental outcomes propose the mechanism of sensing involved the electrostatic interactions of L-Histidine towards AgNPs35, subsequently the CPTPH ligand molecules detached from the surface of AgNPs and eventually gave rise to disappearance of SPR band and rectilinear appearance L-Histidine band. Studies in this regard also suggested the reduction in dielectric constant i.e. alteration of electronic environment surrounding the nanoparticles36 and spatial increase between particles37 resulting in modification of SPR band. Thus, it can be summarized that the CPTPH-AgNPs were particularly selective and sensitive for the L-Histidine amongst other amino acids.
 |
Figure 7: UV-Visible spectra of CPTPH-AgNPs with various amino acids. |
Spectrophotometric Titration of CPTPH-AgNPs with varying concentrations of L-Histidine
Various concentrations of L-Histidine were admixed with CPTPH-AgNPs solution and SPR spectra were recorded. The reduction in intensity of SPR band was noticed when 5.0 μM to 25.0 μM of L-Histidine were added in an increment manner (Fig.8). As L-Histidine concentrations increased by 5.0 μM to 25.0 μM the colour of the CPTPH-AgNPs was started to change from yellow to colourless. Thus, the decrement in absorbance is attributed to the change in the chemical and physical environment near the nanoparticles’ surface38. It is imperative to highlight that since there is no confirmation of the explicit amount of functionalization sites of calix[4]pyrrole on the surfaces of the silver nanoparticles, the current work precludes the determination of the association constant39. Using the calibration curve of concentration vs abso10rbance, the Limit of Detection (LOD) was obtained 6.1 μM employing the LOD = 3.3 × σ / S expressionand Limit of Quantification (LOQ) was found to be 18.5 μM using expression LOQ = 10.0 × σ / S; (σ = standard deviation of the absorbance and S = slope of the curve)40.
 |
Figure 8: Spectrophotometric Titration of CPTPH-AgNPs with increasing concentration of L-Histidine, and linearity curve in inset |
Antimicrobial activity
The medicinal use of colloidal silver extends back to pre-historic India, Egypt, Greece, Rome, Phoenicia, and pre-Columbian civilizations41. Since the time of Charaka, centuries ago, silver and silver-based compounds were used in Indian medicines for therapeutic purposes42. In recent times silver nanoparticles are used widely in the field of medicine to overcome pathogenic attacks.
Table 2: Results Antimicrobial testing (Disc Diffusion Assay)
|
Sr.No. |
Code of Sample |
S. Aureus (in mm) |
B. Subtilis (in mm) |
E. Coli (in mm) |
P. Aeruginosa (in mm) |
A. niger (in mm) |
|
1 |
1 (25 %) |
– |
– |
09.00 |
11.00 |
– |
|
2 |
1 (50 %) |
– |
– |
11.00 |
11.00 |
– |
|
3 |
1 (75 %) |
09.00 |
10.00 |
12.00 |
13.00 |
– |
|
4 |
1 (100 %) |
11.00 |
13.00 |
14.00 |
15.00 |
– |
|
5 |
Chloramphenicol |
18.00 |
22.00 |
27.00 |
25.00 |
N.A. |
|
6 |
Amphotericin B. |
N.A. |
N.A. |
N.A. |
N.A. |
10.00 |
|
Diameter in mm calculated by Hi antibiotic Zone Scale ‘-’ indicates no inhibition of zone, N.A. is Not applicable |
||||||
Numerous antibacterial actions have been reported, irrespective of the fact that the precise mechanism behind the antibacterial activity of silver nanoparticles still seems to be uncertain. In the reported postulate, it has been proposed that AgNPs have the potential to liberate silver ions, electrostatic attraction towards the sulphur and phosphorous of DNA, which causes the deactivation of DNA replication of microbe and eventually kills it43. In another report, it was stated that, upon adhering to the cell surface, silver nanoparticles can aggregate in pockets that develop on the cell walls and AgNPs accumulation can induce cell membrane cleavage44.
 |
Figure 9: Antimicrobial action of CPTPH-AgNPs against the (a) S. Aureus, (b) B. Subtilis, (c) E. Coli, and (d) P. Aeruginosa |
Penetration of AgNPs in bacterial cell wall modifies the structure of cell membrane which imparts denaturation of it was also suggested in one of the reports45. The synthesised CPTPH-AgNPs were studied with S. Aureus and B. Subtilis, P. Aeruginosa and E. Coli and fungi; A. Niger using the disc diffusion method as discussed earlier in the methods. CPTPH-AgNPs have shown plausible antimicrobial activities towards Gram-positive and Gram-negative species and not showing any inhibition for A. Niger (Table-2), but it may be active for other fungi. The study was undertaken with standard antibiotic medicines Chloramphenicol and Amphotericin B.
Conclusion
L-Histidine is a multifunctional essential amino acid, possessing a variety of applications from the human body to pharmaceutical industries, was detected by the functionalized silver nanoparticles selectively and sensitively along with colorimetric results possessing LOD of 6.1 μM and LOQ of 18.5 μM. As prepared stable CPTPH-AgNPs were characterized with UV-Visible spectrophotometer having SPR band at 408 nm, TEM outcomes demonstrated good monodispersed 13 ± 2 nm size AgNPs, having spherical morphology. SAED and EDEX results manifested the crystalline structure with elemental silver. The AgNPs were found to be stable for 120 days and in optimization studies they show 408 nm of absorbance at pH 7.0, and SPR band was intact in the range of temperature range from 10˚ to 30˚ c. The CPTPH-AgNPs colloid solution was assessed against the different microbes incorporating Gram-positive, Gram-negative and fungi, amongst which fair outcomes were attained for bacterial species.
Acknowledgement
We are grateful to the Gujarat Institute of Desert Ecology (GUIDE), Bhuj-Kutch for providing a UV-Visible Spectrophotometer and other facilities. Thanks to Tolani College of Arts and Science, Adipur-Kutch to assist with laboratory instruments, Department of Chemistry, K.S.K.V. Kutch University, Bhuj-Kutch for providing synthesis laboratory and Ganpat University-CARS for sample analysis.
Conflict of Interest
The authors claim that they have no known financial conflicts of interest or close personal relationships that would appear to have impacted the research provided in this study.
Funding Sources
There is no funding sources
References
- Makwana, B. A.; Vyas, D. J.; Bhatt, K. D.; Darji, S.; Jain, V. K. J. A. N., Novel fluorescent silver nanoparticles: sensitive and selective turn off sensor for cadmium ions. 2016, 6 (4), 555-566.
CrossRef - Maity, D.; Gupta, R.; Gunupuru, R.; Srivastava, D. N.; Paul, P., Calix[4]arene functionalized gold nanoparticles: Application in colorimetric and electrochemical sensing of cobalt ion in organic and aqueous medium. Sensors and Actuators B: Chemical 2014, 191, 757-764.
CrossRef - Pomal, N.; Patel, N.; Parikh, J.; Bhatt, K. D. In Strapped Calix[4]Pyrrole: Emerging Trends Based on Calix Protected Metal Nanoparticles, Tailored Functional Materials, Singapore, 2022//; Mukherjee, K.; Layek, R. K.; De, D., Eds. Springer Nature Singapore: Singapore, 2022; pp 457-466.
CrossRef - Jain, V. K., Emerging Trends Based on Calix Protected Metal Nanoparticles. 2016; Vol. 1, Advanced Materials: TechConnect Briefs 2016, p 145-148.
- Keyur D. Bhatt, A. D., Krunal Modi, Anita Kongor, Coupling Reactions by Highly Efficient Octacalix[4] Pyrrole Wrapped Scrupulous Nano-Palladium Catalyst. Biointerface Research in Applied Chemistry 2021, 11 (1), 7632-7645.
CrossRef - Bhatt, K. D.; Vyas, D. J.; Makwana, B. A.; Darjee, S. M.; Jain, V. K.; Shah, H., Turn-on fluorescence probe for selective detection of Hg(II) by calixpyrrole hydrazide reduced silver nanoparticle: Application to real water sample. Chinese Chemical Letters 2016, 27 (5), 731-737.
CrossRef - Pomal, N. C.; Bhatt, K. D.; Modi, K. M.; Desai, A. L.; Patel, N. P.; Kongor, A.; Kolivoška, V., Functionalized Silver Nanoparticles as Colorimetric and Fluorimetric Sensor for Environmentally Toxic Mercury Ions: An Overview. Journal of Fluorescence 2021, 31 (3), 635-649.
CrossRef - Kongor, A.; Panchal, M.; Athar, M.; Vora, M.; Verma, N.; Pandya, A.; Jha, P. C.; Bhadresha, K.; Rawal, R.; Jain, V., Colorimetric and electrochemical sensing of As(III) using calix[4]pyrrole capped gold nanoparticles and evaluation of its cytotoxic activity. Journal of Inclusion Phenomena and Macrocyclic Chemistry 2020, 98 (1), 29-41.
CrossRef - Moulaee, K.; Neri, G., Electrochemical Amino Acid Sensing: A Review on Challenges and Achievements. 2021, 11 (12), 502.
CrossRef - Aliu, E.; Kanungo, S.; Arnold, G. L. J. A. o. T. M., Amino acid disorders. 2018 2018, 6 (24), 471.
CrossRef - Derave, W.; Everaert, I.; Beeckman, S.; Baguet, A., Muscle Carnosine Metabolism and β-Alanine Supplementation in Relation to Exercise and Training. Sports Medicine 2010, 40 (3), 247-263.
CrossRef - Poon, I. K.; Patel, K. K.; Davis, D. S.; Parish, C. R.; Hulett, M. D. J. B., The Journal of the American Society of Hematology, Histidine-rich glycoprotein: the Swiss Army knife of mammalian plasma. 2011, 117 (7), 2093-2101.
CrossRef - Dahl, T. A.; Midden, W. R.; Hartman, P. E. J. P.; photobiology, Some prevalent biomolecules as defenses against singlet oxygen damage. 1988, 47 (3), 357-362.
CrossRef - Moriguchi, T.; Takai, J., Histamine and histidine decarboxylase: Immunomodulatory functions and regulatory mechanisms. 2020, 25 (7), 443-449.
CrossRef - M. Korhonen, A. V., P. Huhtanen, Effect of Protein Source on Amino Acid Supply, Milk Production, and Metabolism of Plasma Nutrients in Dairy Cows Fed Grass Silage. Journal of Dairy Science 2002, 85 (12), 3336–3351.
CrossRef - Wade, A. M.; Tucker, H. N., Antioxidant characteristics of L-histidine 11The work described in this manuscript was partially sponsored and funded by Cytos Pharmaceuticals, LLC. The Journal of Nutritional Biochemistry 1998, 9 (6), 308-315.
CrossRef - Brosnan, M. E.; Brosnan, J. T., Histidine Metabolism and Function. The Journal of Nutrition 2020, 150 (Supplement_1), 2570S-2575S.
CrossRef - Holeček, M., Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. 2020, 12 (3), 848.
CrossRef - Zhang, Z.-H.; Wei, F.; Vaziri, N. D.; Cheng, X.-L.; Bai, X.; Lin, R.-C.; Zhao, Y.-Y., Metabolomics insights into chronic kidney disease and modulatory effect of rhubarb against tubulointerstitial fibrosis. Scientific Reports 2015, 5 (1), 14472.
CrossRef - Geliebter, A. A.; Hashim, S. A.; Van Itallie, T. B., Oral L-histidine fails to reduce taste and smell acuity but induces anorexia and urinary zinc excretion. The American Journal of Clinical Nutrition 1981, 34 (1), 119-120.
CrossRef - Deepa, A.; Srinivasadesikan, V.; Lee, S.-L.; Padmini, V., Highly selective and sensitive detection of histidine by naked eye and fluorimetric method in aqueous medium via hydrogen bonding. Journal of Photochemistry and Photobiology A: Chemistry 2020, 400, 112615.
CrossRef - Huang, P.; Li, J.; Song, J.; Gao, N.; Wu, F., Silver nanoparticles modified with sulfanilic acid for one-step colorimetric and visual determination of histidine in serum. Microchimica Acta 2016, 183 (6), 1865-1872.
CrossRef - Akar, A.; Aydogan, A., Synthesis of meso-tetra acid and ester functionalized calix[4]pyrroles. 2005, 42 (5), 931-934.
CrossRef - Liu, Y.; Liu, J.; Yang, H.; Liu, K.; Miao, R.; Peng, H.; Fang, Y., Dynamic covalent bond-based hydrogels with superior compressive strength, exceptional slice-resistance and self-healing properties. Soft Matter 2018, 14 (39), 7950-7953.
CrossRef - Lai, F.; Yang, J.; Huang, R.; Wang, Z.; Tang, J.; Zhang, M.; Miao, R.; Fang, Y., Nondestructive Evaluation of Fish Freshness through Nanometer-Thick Fluorescence-Based Amine-Sensing Films. ACS Applied Nano Materials 2021, 4 (3), 2575-2582.
CrossRef - Jorgensen, J. H.; Turnidge, J. In Susceptibility Test Methods: Dilution and Disk Diffusion Methods*, 2015.
CrossRef - Johnson, E. M.; Cavling-Arendrup, M., Susceptibility Test Methods: Yeasts and Filamentous Fungi. In Manual of Clinical Microbiology, 2015; pp 2255-2281.
CrossRef - Darjee, S. M.; Bhatt, K. D.; Panchal, U. S.; Jain, V. K., Scrupulous recognition of biologically important acids by fluorescent “turn off-on” mechanism of thaicalix reduced silver nanoparticles. Chinese Chemical Letters 2017, 28 (2), 312-318.
CrossRef - Zang, W.; Chen, X.; Boulos, R. A.; Toster, J.; Raston, C. L., Hydrogen induced p-phosphonic acid calix[8]arene controlled growth of Ru, Pt and Pd nanoparticles. Chemical Communications 2014, 50 (96), 15167-15170.
CrossRef - Kongor, A.; Panchal, M.; Athar, M.; Jha, P. C.; Jhala, D.; Sindhav, G.; Shah, N.; Jain, V. K., Selective fluorescence sensing of Cu(II) ions using calix[4]pyrrole fabricated Ag nanoparticles: A spectroscopic and computational approach. Journal of Molecular Liquids 2018, 269, 467-475.
CrossRef - Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V., Identifying Trends in Gold Nanoparticle Toxicity and Uptake: Size, Shape, Capping Ligand, and Biological Corona. ACS Omega 2019, 4 (1), 242-256.
CrossRef - Bhatt, K. D.; Vyas, D. J.; Makwana, B. A.; Darjee, S. M.; Jain, V. K., Highly stable water dispersible calix[4]pyrrole octa-hydrazide protected gold nanoparticles as colorimetric and fluorometric chemosensors for selective signaling of Co(II) ions. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2014, 121, 94-100.
- Vyas, G.; Bhatt, S.; Paul, P., Synthesis of Calixarene-Capped Silver Nanoparticles for Colorimetric and Amperometric Detection of Mercury (HgII, Hg0). ACS Omega 2019, 4 (2), 3860-3870.
CrossRef - Li, X.; Lenhart, J. J.; Walker, H. W., Aggregation Kinetics and Dissolution of Coated Silver Nanoparticles. Langmuir 2012, 28 (2), 1095-1104.
CrossRef - Wangoo, N.; Bhasin, K. K.; Mehta, S. K.; Suri, C. R., Synthesis and capping of water-dispersed gold nanoparticles by an amino acid: Bioconjugation and binding studies. Journal of Colloid and Interface Science 2008, 323 (2), 247-254.
CrossRef - Evanoff Jr., D. D.; Chumanov, G., Synthesis and Optical Properties of Silver Nanoparticles and Arrays. 2005, 6 (7), 1221-1231.
CrossRef - Zhang, Y. J., Comparing the interparticle coupling effect on sensitivities of silver and gold nanoparticles. Journal of Quantitative Spectroscopy and Radiative Transfer 2012, 113 (8), 578-581.
CrossRef - Lee, K.-S.; El-Sayed, M. A., Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. The Journal of Physical Chemistry B 2006, 110 (39), 19220-19225.
CrossRef - Perret, F.; Tauran, Y.; Suwinska, K.; Kim, B.; Chassain-Nely, C.; Boulet, M.; Coleman, A. W., Molecular Recognition and Transport of Active Pharmaceutical Ingredients on Anionic Calix[4]arene-Capped Silver Nanoparticles. Journal of Chemistry 2013, 2013, 191828.
CrossRef - Wenzl, T.; Haedrich, J.; Schaechtele, A.; Robouch, P.; Stroka, J., Guidance Document for the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food. 2016.
- Silva, L. P.; Silveira, A. P.; Bonatto, C. C.; Reis, I. G.; Milreu, P. V., Chapter 26 – Silver Nanoparticles as Antimicrobial Agents: Past, Present, and Future. In Nanostructures for Antimicrobial Therapy, Ficai, A.; Grumezescu, A. M., Eds. Elsevier: 2017; pp 577-596.
CrossRef - Galib; Barve, M.; Mashru, M.; Jagtap, C. Y.; Patgiri, B.; Prajapati, P. K. J. J. o. A.; Medicine, I., Therapeutic potentials of metals in ancient India: A review through Charaka Samhita. 2011, 2, 55 – 63.
CrossRef - Bapat, R. A.; Chaubal, T. V.; Joshi, C. P.; Bapat, P. R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. J. M. S.; C, E., An overview of application of silver nanoparticles for biomaterials in dentistry. 2018, 91, 881-898.
CrossRef - Liao, C.; Li, Y.; Tjong, S. C. J. I. j. o. m. s., Bactericidal and cytotoxic properties of silver nanoparticles. 2019, 20 (2), 449.
CrossRef - Yin IX, Z. J., Zhao IS, Mei ML, Li Q, Chu CH, The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int J Nanomedicine 2020, 15, 2555-2562.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










