A Facile Synthesis and Characterization of New N-P-K Fertilizer Fortified with Tri -Micronutrient Matrix and its Application for Optimal Plant Augmentation
Department of Chemistry, Institute of Science, GITAM University Visakhapatnam-530045,A.P,India.
Corresponding Author E-mail: vnutulap@gitam.edu
DOI : http://dx.doi.org/10.13005/ojc/370626
Article Received on : 18-Oct-2021
Article Accepted on :
Article Published : 07 Dec 2021
Reviewed by: Dr.Areej Ali Jarullah
Second Review by: Dr. Likaa Khalid
Final Approval by: Dr. Haresh Thakellapalli
At present in agricultural techniques engaged the optimal nutrient supply is very imperative factor for elevated crop yield and all essential plant macro and micronutrients. Micronutrients play vital role in photosynthesis. For balanced crop nutrition micronutrient support is essential. Micronutrient fertilizer required in small quantities by plants and occupy vital role in physiology of plant kingdom. Proven fact is decisive plant functions are over involved if enough micronutrients are engaged. In this paper the author developed a new N-P-K fertilizer fortified with tri -micronutrient matrix and its application for optimal Plant growth. The author developed and analyzed hundred percent water soluble active fertilizer(15-15-15) with three important micronutrients (-3.4 0.5%Zn, 0.5%Fe, 0.5% Mg). Pot experiments were conducted with and without proposed fertilizer on Solanum lycopersicum(tomato) seeds. The proposed enhanced efficiency fertilizer with tri micronutrient matrix showed better plant growth as compared to normal untreated fertilizer in low nutrient soil field.
KEYWORDS:Micronutrient Matrix; NPK fertilizer; Pot Experiments; Plant Growth; Solanum lycopersicum seeds
Download this article as:| Copy the following to cite this article: Venugopal N. V. S, Mohana Rao G. N. V. A Facile Synthesis and Characterization of New N-P-K Fertilizer Fortified with Tri -Micronutrient Matrix and its Application for Optimal Plant Augmentation. Orient J Chem 2021;37(6). |
| Copy the following to cite this URL: Venugopal N. V. S, Mohana Rao G. N. V. A Facile Synthesis and Characterization of New N-P-K Fertilizer Fortified with Tri -Micronutrient Matrix and its Application for Optimal Plant Augmentation. Orient J Chem 2021;37(6). Available from: https://bit.ly/3pvm4V7 |
Introduction
Food security is one of the world’s big challenges with huge demand forecast. Every year a series of agricultural products, drink and food products for human consumption and animal feed are formed. Climatic conditions and total geography have big impact on agricultural use of available land. Various materials added to the soil in order to supply different chemical elements for better soil fertility. Fertilizers are needed for receiving increase in crop growth and yield. Macronutrients such as Nitrogen, Phosphorous and Potassium were recognized for the supply of important nutrients to all crops. At present more chemical fertilizers used by farmers to produce higher yield and augment reasonable efficiency. The use efficiency of N-P-K is about 30–60%; 30–50% and 10–20%, respectively and these reflect low macronutrients use efficiency for crops. The fertilizers not in use by crops instead entering in to the environment is the pressing concern to the world.
Visual symptoms on crops always reveal micronutrients deficiency and it can be tested from plant tissues and soil. Micronutrients like Iron, Zinc and Magnesium have important role for plant growth. Iron is very important for biological process and cell growth in plants. The main component of enzymes indispensable for chlorophyll synthesis, photosynthesis etc is Iron. The role of Zinc is vital for plant hormone balance and auxin activity. Magnesium is the central core of the chlorophyll molecule in plant tissue. Shortage of chlorophyll results low plant growth if Magnesium is deficient. Magnesium deficiency leads to boost contribution of production and also diminishes cost-effective efficiency and augments environmental pollution.1-2
The recent scenario was in the preparation and application of nanofertilizers. Nanofertilizers help increase the effectiveness of fertilizer, enhance yield and excellence of crops, diminish detrimental effects of chemical fertilizers on environment and develop a green and sustainable agriculture3-12.By coating urea into nanofilm(nanourea) has been triumphant using it for Canola13. To enhance yield and reduce nitrate leaching, Nano Nitrogen fertilizer synthesized by coating Urea with Sulfur and nano Nitrogen chelate.Nano Nitrogen was effectively applied for potato. The current research in progress by many researchers in the world was to develop Complex NPK nanofertilizer. Wu and Liu investigated the encapsulation of NPK fertilizer into chitosan and coating the outer by poly (acrylic acid-co-acrylamide). Complex NPK nanofertilizer was prepared by trapping the fertilzer in polyacrylic hydrogel and later the study of the NPK slow release control of the nanofertilizer14. For loading nutrients to various crops materials like Chitosan and its nanoparticles etc were functional as a useful matrix. Complex NPK fertilizer was encumbered into chitosan nanoparticles, and chitosan nanoparticles with polymethacrylic acid (MMA)15-19 Therefore from the past forty years Chemical fertilizers have been crucial to the growth of world agricultural production.
Materials and methods
The various raw materials used in the current study were given in table1. All other chemicals used were of analytical grade.
 |
Table 1: Raw materials |
The above-mentioned raw materials of fertilizers grade were analyzed by using existing methods defined in Fertilizer control order (FCO) procedures to know the nutrient contents accordingly final product formulation prepared. The methods of analysis used for the analysis of raw materials were given in table 2.
 |
Table 2: Methods of analysis used for the analysis of raw materials |
Instrumentation
Inductively coupled plasma optical emission spectrometry (ICP-OES), Perkin-Elmer (Optima7000DV) was preferred for elemental analysis. For macronutrients, Auto analyzer (Skalar) was used. Analytical balance, Top load balance (Sartorius), Hot air oven (Tempo),etc were used in the study.
Methods
All the standard solutions were standardized as per procedure before proceeding to analysis. Performed the calibration to weighing balances and hot air oven at the time of analysis. Blank estimation was performed for all the parameters on the reagents and accordingly correction given to the samples analysis. Duplicate determination was performed for each parameter in the analysis and average was taken as final result.
Preparation of N-P-K fertilizer fortified with tri -micronutrient grade
Each substance –Mono Ammonium Phosphate, Ammonium Sulphate, Urea, Potassium Nitrate, Zinc EDTA, Ferrous Sulphate hepta hydrate and Magnesium Sulphate hexa hydrate have taken 100gm grounded individually with help of Mortar and pestle and kept for drying in a vacuum desiccators for 24 hours. after 24 hours the substances are properly kept in self sealed covers to avoid moisture absorption. Three replicate mixtures were prepared separately and each replicate consists of 25.0gm of Mono Ammonium Phosphate, 19.29gm of Ammonium Sulphate, 10gm of Urea, 33.71gm of Potassium Nitrate, 4.17gm of Zinc EDTA, 2.63gm of Ferrous sulphate hepta hydrate and 5.21gm of magnesium sulphate hexa hydrate. All the mixtures are finely grounded and mixed for 20min in a mixer of Preeti make, model no MG 198 (1300 watts) with 4000rpm by using small jar of capacity 700ml. All the three replicate mixtures are dried in a vacuum desiccators for 24 hours to remove surface moisture absorbed during the preparation process.
Results and Discussion
Initially all the raw materials were analyzed and the results are given in table 3.
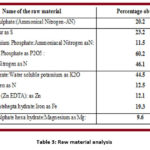 |
Table 3: Raw material analysis |
A new formulated 100% water soluble fertilizer with three important micronutrients (15-15-15-3.4 0.5%Zn, 0.5%Fe, 0.5% Mg) was developed and analyzed. The N-P-K fertilizer fortified with tri -micronutrient product was shown in figure 1.
 |
Figure 1: N-P-K fertilizer fortified with tri -micronutrient. |
Analysis
Total Nitrogen and Nitrate nitrogen are analyzed by macro Kjedhal distillation method as per FCO 1985 procedures.
Water soluble Potassium was analyzed by STPB (sodium tetra phenyl boran) method prescribed in FCO1985. For this taken 2.5gm sample and dissolved in Ammonium oxalate, and from that required aliquot taken and added 2mL 20% NaOH , 5mL HCHO and 25mL STPB reagent and made upto 100mL mark with distilled water. Filtered through no. 42 Whatman filter paper. 50ml filtrate was taken in 150mL conical flask titrated against Cetyl trimethyl ammonium bromide. Blank and standard KH2PO4 determination was also done and accordingly correction given.
Sulphate sulphur was determined in the samples by digesting the samples with Concentrated HCl, filtered and precipitated as Barium sulphate with Barium chloride solution, dried at
250 0C, weighed and calculated as Sulphur.
Ammoniacal nitrogen (AN), Urea Nitrogen (UN), Water Soluble phosphate(P2O5) were analyzed by segmented flow analyzer also called Auto analyzer.
Sample preparation: Three replicates were analyzed for physical parameters, nutrients and heavy metals present in the new proposed fertilizer grade. Known quantity of the sample dissolved in distilled water, filtered, and analyzed on segmented flow analyzer (SKALAR SAN++ system).
Principles involved in the analysis on the auto analyzer:
AN determination
The reaction involved is Berthlot reaction in which Ammonia reacts with sodium hypo chlorite in presence of buffer and forms monochloramine which reacts with salicylate to form 5-aminosalicylate. A green colored complex is formed after oxidation and oxidative coupling which is then measured at 660nm.
WSP2O5 determination
The sample is mixed with ammonium heptamolybdate solution and forms molybdophosphoricaicd which reacts with metavanadate to form stable yellow coloured vanadomolybdophosphoric acid, measured at 420nm.
UN determination:The sample is mixed with diacetyl monoxime at 90 0C, forms coloured complex, which is further intensified by the addition of thiosemicarbazide and an acid reagent is used to increase the rate of colour formation, measured at 520nm.
A combined stock standard solution of 10,000ppm P2O5, 4000ppm AN, 4660ppm UN prepared by taking appropriate weights of AR grade NH4Cl, KH2PO4, and Urea. From this stock solution, three working standards (S1, S2, S3) prepared, in which S1 contains 500ppm P2O5, 233ppm UN, 200ppm AN; S2 contains 1000ppm P2O5, 466ppm UN, 400ppm AN; and S3 contains 1500ppm P2O5, 699ppm UN, 600ppm AN. Analyzer was calibrated with above 3 working standards in first order reaction mode. Correlation coefficient for all three parameters is above 0.999 and RSD is <0.01% (shown in figure: 2,3 &4)
 |
Figure 2: Ammoniacal Nitrogen Calibration graph on auto analyser |
 |
Figure 3: Phosphate Calibration graph on auto analyser |
 |
Figure 4: Urea Nitrogen Calibration graph on auto analyser |
Micronutrients- Zn, Fe and Mg analyzed on ICP OES (OPTIMA 7000DV).
The samples were strongly digested with HCl and HNO3 and then diluted with Millipore ultra-pure distilled water (conductivity<0.1microsiemens /cm2). Calibration was done with three multi element working standards of 10,50,100ppm which were prepared from stock 1000ppm ICP multi element standard solution IV (Merck, certipur). Same acid back ground maintained for working standards used for calibration. The wave lengths selected for analysis of Fe is 238.204nm, Zn is 213.857nm and Mg is 278.297nm. Correlation coefficient was above 0.999 and RSD was less than 1.0%.
Heavy metals-As , Pb & Cd were analyzed on ICP OES (OPTIMA 7000DV).
The samples were strongly digested with HCl and HNO3 of trace metal grade, then diluted with Millipore ultra-pure distilled water. Calibration was done with three multi element working standards of 0.25,0.5 and 1.0ppm which were prepared from stock 100ppm ICP multi element standard solution X (Merck, certipur).). Same acid back ground maintained for working standards used for calibration. The wave lengths selected for analysis of As is 188.979nm, Pb is 220.350nm and Cd is 226.50nm. Correlation coefficient was observed above 0.999 and RSD was less than 5.0%.
 |
Table 4: Analysis of N-P-K fertilizer fortified with tri -micronutrient |
Application of the proposed fertilizer to Solanum lycopersicum seeds :
The investigation of Solanum lycopersicum plants under controlled conditions was carried without off-putting effects of various environmental aspects. Pot experiments were conducted with and without the proposed fertilizer. In one pot soil weight of 955gm and 100ml of water and gently mixed and on the top of the soil 0.03gm of Solanum lycopersicum seeds applied uniformly. In another pot, soil weight of 954gm and 100ml of 1% proposed fertilizer in water are gently mixed to get uniformity in the soil and on the top of the soil 0.03gm of Solanum lycopersicum seeds applied uniformly. The growth pattern was shown in figure 5.
 |
Figure 5: Growth pattern of Solanum lycopersicum |
All crops require important macronutrients such as N-P-K. Fertilizers are crucial to different crops, endow with obligatory nutrients for crop growth, and increase crop yield and quality of the crop. N-P-K fertilizer fortified with tri -micronutrient matrix and its application for optimal Plant growth shown best results and more crop yield. The proposed fertilizer showed better plant growth as compared to normal low nutrient soil field.
Acknowledgement
The author wish to thank the management of GITAM (Deemed to be University), Visakhapatnam, Andhra Pradesh, India for supporting this work
Conflict of Interest
The authors declare that there are no conflicts of interest.
Founding Sources
There are no funding sources.
References
- Sun, B.; Zhang, L.; Yang, L.; Zhang, F.; Ambio., 2012, 41, 370.
CrossRef - Trenkel,M.M, Controlled release and stabilized fertilizer in agriculture (International Fertilizer Industry Association, Paris.2010.
- Corredor,E.; Testillano, P.S.; José Coronado, M.; González-Melendi, P.; Fernández-Pacheco, R.; Marquina,C.; Ricardo Ibarra, M.; De la Fuente, J.M.; Rubiales, D.; De Luque, A.P.; Carmen Risueño, M.; 2009, BMC Plant Biol.,2009,9, 1.
- Ghormade, V.; Deshpande, M.V.;Paknikar, K.M.; Biotechno.,,2011, 29, 792.
CrossRef - Huang, S.; Wang, L.; Liu, L.; Agron. Sustain. Dev.,2015, 35, 369–400.
CrossRef - Khodakovskaya, M.;Dervishi, E.;Mahmood, M.; Xu, Y.; Z. Li, F.; Watanabe, A.S.; ACS Nano., 2009,3, 322.
CrossRef - Liu, R.; Lal, R.;Sci Rep.,2014,4, 5686.
CrossRef - Prasad,T.N.V.K.V.; Sudahka, P.; Sreenivasulu, Y.; Latha, P.;Munaswamy, V.; Raja Reddy, K.; Sreeprasat, T.S.; Panikkanvalappil, S.R.; Thalappil, P.;J.Plant Nutr. 2012,35, 905.
CrossRef - Servin, A.; Elmer, W.; Mukherjee, A.; Hamdi, H.; White, J.C.; Bindranban, P.; Dimkpa, C., J. Nanopart. Res ,2015,17, 92.
- Tarafdar, J.C.; Raliya, R., Mahawar, H.;Agric Res., 2014,3,257–262.
CrossRef - Wu, L.; Liu, M.; Carbohydr. Polym.,2008,72, 240.
CrossRef - Zareabyaneth, H.; Bayatvarkeshi, M.; cropEnviron. Earth Sci.,2015,74, 3385.
CrossRef - De Rosa, M.C.; Monreal, C.; Schitzer, M.; Walsh, R.; Sultan, Y.; Nanotechnol., 2010,5, 9.
CrossRef - Teodorescu, M.; Lungu, A.; Stanescu,P.O.; Neamtu,C.;Ind. Eng. Chem. Res.,2009,48, 6527.
CrossRef - Corradini, E.; de Moura, M.R.; Mattoso, L.H.C.;Polym. Letters.,2010, 4, 509.
CrossRef - Ha, N.M.C.; Nguyen, T.; Wang, H.; Res Chem Intermed., 2019,45, 51–63.
CrossRef - Phelan, P.; Moloney, A. P.; McGeough, E. J.; Humphreys, J.; Bertilsson, J.; O’Riordan, E.G.; O’Kiely, P.; Critical Reviews in Plant Sciences,2015, 34, 281-326.
CrossRef - Garde-Cerdan,T.;Santamaría, P.; Rubio-Breton, P.; Gonzalez-Arenzana, L.;Lopez-Alfaro, I.; Lopez, R.; Food Sci. Technol,2015,60, 684-689.
CrossRef - Fernández-Escobar, R.; Antonaya-Baena, M.F.; Sánchez-Zamora, M.A.; Molina- Soria,C.; Scientia Hortic.,2014,167, 1-4.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










