Synthesis and Spectral studies of Ru (II) complexes with Macrocyclic Ligands
Ashish Rajak1, Arpit Srivastava1, Ramakant1, Subhash Chandra Shrivastava1*, Ranjeet Singh Chauhan2, Uday Singh Patel3 and Shekhar Srivastava1
1Department of Chemistry, University of Allahabad, Prayagraj-211002 UP, India.
2Department of Chemistry, Gochar Mahavidyalaya, Rampur Maniharan, Saharanpur-247451 UP, India.
3Department of Chemistry, D A V (P G) College Knapur-208001 UP, India.
Corresponding Author E-mail: subhash.chem.au@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370401
Article Received on : 26-Jun-2021
Article Accepted on :
Article Published : 23 Jul 2021
Reviewed by: Dr. Arivazhagan Ganesh
Second Review by: Prof. Dr. Jasim Alshawi
Final Approval by: Dr. Maged S. Al-Fakeh
Fifteen Ru (II) complexes of the type [RuCl2(L)] (where L= N4 donor macrocyclic ligands) have been synthesised by reaction of [(RuCl2(DMSO)4] with fifteen macrocyclic Schiff base ligands containing N4 donors groups. The prepared fifteen [RuCl2(L)] complexes were characterised by elemental analysis, molar conductivity, UV-visible, IR, X- ray photoelectron spectra (XPS) and magnetic susceptibility measurementsand an octahedral geometry was proposed for all these complexes.
KEYWORDS:Magnetic Susceptibility Measurement; N4 Macrocyclic Shiff Base Complexes; Ru (II) Complexes, Elemental Analysis; UV-visible
Download this article as:| Copy the following to cite this article: Rajak A, Srivastava A, Ramakant R, Shrivastava S. C, Chauhan R. S, Patel U. S, Srivastava S. Synthesis and Spectral studies of Ru (II) complexes with Macrocyclic Ligands. Orient J Chem 2021;37(4). |
| Copy the following to cite this URL: Rajak A, Srivastava A, Ramakant R, Shrivastava S. C, Chauhan R. S, Patel U. S, Srivastava S. Synthesis and Spectral studies of Ru (II) complexes with Macrocyclic Ligands. Orient J Chem 2021;37(4). Available from: https://bit.ly/3zm9878 |
Introduction
During past few decades the chemistry of macrocyclic of metal have been developed very extensively due to its applications in coordination chemistry1,2 bioinorganic chemistry3 catalysis 4-6 organometallic chemistry; 7 and biocoordination chemistry 8. Many macrocyclic ligands e-g. Robson type tetraiminodiphenol macrocyclic ligands 9,10 N4S2 donor macrocyclic ligands 11; crown ethers; porphyrins; saturated and unsaturated polyamine 6-12; polyazamacrocycles 18; have been synthesised.
A number of schiff base macrocyclic ligands have been reported 14. In which many macrocyclic ligands have been synthesised by the template condensation reaction 15. Several transition metal ion complexes in living organisms work as enzymes or carriers in macrocyclic ligand environment 16 and used as modelling on the active sites of metalloenzymes 17.
Various macrocyclic metal complexes have been used as detecting tumor lesions18 ; as in labeling monoclonal antibodies with radioactive models 19 as in metal ion separation 20; as anticancerous 21; as toxicity against bacterial fungal growth 22; as photosenstizer 23; in photosynthesis and dioxygen transport 24 ; as catalyst 25-27; as pharmalogical agent; 28,29 cancer diagonsis 30-32; as therapeutic radiotherapeutic 33; as antitumour 34; as potential medical applications 35; as environmental importance 36; as RNA cleavage catalyst 37; and as NMR shift and relaxation agents 38.
Few comprehensive reviews on macrocyclic ligands, their metal complexes and applications have been also published in last few years 39. Many ruthenium (II) & (III) Schiff base and macrocyclic Schiff base complexes have been extensively studied in last few years due to their importance as biochemical and analytical reagents 40. But ruthenium (II) macrocyclic Schiff base complexes are less known e.g.
Spectroscopic data studies and structures of trans–Ru(II) and Ru(III) bis (cyanide) complexes sustained by tetradentate macrocyclic tertiary amine ligand 41; N- macrocyclic complexes of Ru (II) & Ru (III); tetraaza macrocyclic Ru(II) complexes: synthesis, spectral and catalytic studies 42; the versatile Ru II / Ru III teraaza macrocyclic complexes and their nitrosyl derivative [43]; Ru(II) & (III) complexes with cyclam and related species 44; Ru (II) / (III) and oxovanadiun (IV) complexes with macrocyclic schiff base complexes; Ru (II) complexes of a new tetrapyrazolic macrocyclic; some new polynucleating ligands containing Ru (II) complexes incorporating terpyridyl and macrocyclic aza-crown binding sites. Recently some reviews on macrocyclic ligands have been also published 45.
Shankar et al. 46 have been synthesised various ruthenium (II) complexes with macrocyclic Schiff base ligands derived from orthophthaldehyde and different diamines. Characterised and used as catalysts for the reduction of pralidoxime to its amino derivatives. In this present communication, fifteen macrocyclic schiff base ligands will be synthesie by condensation reaction of 2,5-diformyl furan or furil or phenanthrene 9,10-dione with different aliphatic diamines NH2(CH2)nNH2 i.e. NH2CH2CH2NH2 or NH2CH2CH2CH2NH2 or NH2CH2CH2CH2CH2NH2 or NH2CH2CH2CH2CH2CH2NH2 or NH2CH2CH2CH2CH2CH2CH2NH2 and their ruthenium (II) metal complex through template synthesis in 2:2:1 molar ratio respectively.
Experimental
RuCl3.3H2O (Johnson Matthey & Co, Ltd; triphenylphosphine Merck, Mumbai); ethanol (Merck, Mumbai); dichloromethane (Aldrich) and acetone (Aldrich ) were AR Grade and used after purification and dried. Solvents were distilled from relevent drying agents immediately in advance of use. [RuCl2 (PPh3)4] was prepared by published method.
Melting points were determined by using in sealed capillary tube on Melting point apparatus. The C and H were determined by CDRI, LUCKNOW, INDIA. Nitrogen and chlorine were determined by Kjeldahl’s and Volhard’s methos respectively. Molar conductivity was measured on Elico-CM 82 conductivity bridge in acetone at room temperature. IR spectra were recorded on Perkin-Elmer 1000 IR spectrometer using KBr/CsI pellets. Electronic spectral measurements were recorded on Elico SL159 spectrophotometer in the range 300-1000 nm. Magnetic measurements were carrid out a Cahn 2000 electo balance by Faraday Method using Hg[Co(SCN)4] as calibrant. Pascal constants were used for diamagnetic corrections. Kratos analytical Axis Supra ESCA i.e. X-ray photoelectron spectroscopy i.e. XPS instrument equipped with monochromatised Alkα (1486.6 eV) source is used. All the peaks were rectified for charging with reference to C1S peak 284.8 eV and counterd with Shirley background and a union of Gavssian and Lorentzian line- shapes, using ESCApe software.
2,5- diformylfuran or furil or phenanthrene 9,10- dione was mixed in dry ethanolic solution of NH2(CH2)nNH2 (2 mmol) (where (2 mmol) n=2 or 3 or 4 or 5 or 6). and refluxed for 2 hrs for preparation of ligands L1 to L15). In this resulting each solution poured [RuCl2(PPh3)4 (1mmol ) i.e. 1:1 molar ratio and again refluxed for about 2 hrs. The resulting precipitate was filtered and recrystallised by benzene: pet-ether (9:1) ratio (Figs. 1-3).
 |
Figure 1: Preparation of [RuCl2L1-5] complexes |
 |
Figure 2: preparation of [RuCl2 (L6-10)] complexes. |
 |
Figure 3: Preparation of [RuCl2(L11-15)] complexes |
Results and Discussion
Fifteen tetraaza macrocycliccomplexes containing Ru(II) were synthesised by interaction of [RuCl2(PPh3)4] with fifteen macrocyclic Schiff base ligands (L1 to L15). The complex were soluble in DMF, DMSO and chloroform. The elemental analysis (C, H, N and Cl) were consistent within ±0.5% with the suggested structure of the complexes. The molar conductance values were found to below (12.0-20.0 ohm-1 cm2 mol-1) recommending the non electrolytic nature of the complexes 47.
In these entire [RuCl2L1-15] complexes IR band due to νC=N was shifted towards lower side about 20-40 cm-1 with respect to the ligand spectra and was obtained in the range of 1600- 1580 cm-1. A low intensity band in the region of 520-500 cm-1 were observed due to νRu-N vibration confirm that ligands coordinate to Ru metal ion through nitrogen of C=N group in all these complexes 48. Only one band was observed in the range of 320-300 cm-1 in IR spectra of all these complexes indicating the presence of two chloride ions in trans position around ruthenium ion [49]. All the Characteristic IR bands were also observed due to aromatic rings in the expected region in all these [RuCl2L1-15] complexes 49.
The ground state of Ru (II) t2g6 electronic configuration is 1A1g. For octahedral Ru (II) complexes four electronic transition are possible i.e 1A1g→3T1g; 1A1g→3T2g; 1A1g→1T1g and 1A1g→1T2g. In each complexes of [RuCl2 (L1-15)] two electronic bands were observed in the region 200-530 nm. These electronic bands have been assigned to the spin allowed 1A1g-1T1g transition at lower wavelength (460-520 nm) based on molar extinction coefficient. The another high intensity band at 280-290 nm region has been assigned due to charge transfer transition originated from the excitation of an electron from the metal t2g level to the unfilled molecular orbital derived from the π* level of the ligands. All these [RuCl2L1-15] complexes have shown negative magnetic susceptibility and magnetic moment values below then 1.0 BM at room temperature corresponding to diamagnetic nature.
Table 1: Physical and Analytical data of [RuCl2L1-15)] Complexes
|
Sr. No |
Complexes |
% found (Calculated) |
^M Ohm-1 cm2 mol-1 |
|||
|
C |
H |
N |
Cl |
|||
|
1 |
[RuCl2.L1)] |
41.4 |
3.2 |
11.6 |
15 |
10 |
|
-41.5 |
-3.3 |
-11.8 |
-15 |
|||
|
2 |
[RuCl2.L2)] |
43.4 |
4 |
11 |
14.2 |
12 |
|
-43.5 |
-4 |
-11.2 |
-14.3 |
|||
|
3 |
[RuCl2.L3)] |
42.6 |
4.4 |
10.6 |
13.4 |
18 |
|
-42.7 |
-4.5 |
-10.6 |
-13.5 |
|||
|
4 |
[RuCl2.L4)] |
47.6 |
5 |
10 |
12.6 |
16 |
|
-47.8 |
-5 |
-10.1 |
-12.8 |
|||
|
5 |
[RuCl2.L5)] |
50.4 |
5.4 |
9.4 |
12 |
10 |
|
-50.5 |
-5.4 |
-9.4 |
-12 |
|||
|
6 |
[RuCl2.L6)] |
54.2 |
3.6 |
10.5 |
13.4 |
12 |
|
-54.4 |
-3.7 |
-10.5 |
-13.4 |
|||
|
7 |
[RuCl2.L7)] |
56 |
4.2 |
10 |
12.6 |
14 |
|
-56 |
-4.3 |
-10 |
-12.7 |
|||
|
8 |
[RuCl2.L8)] |
69 |
5.6 |
11.4 |
14.6 |
16 |
|
-69.2 |
-5.7 |
-11.5 |
-14.6 |
|||
|
9 |
[RuCl2.L9)] |
30.6 |
4 |
7 |
9 |
12 |
|
-30.7 |
-4.4 |
-7.1 |
-9 |
|||
|
10 |
[RuCl2.L10)] |
59.8 |
3.2 |
11.6 |
15 |
18 |
|
-59.9 |
-3.3 |
-11.8 |
-15 |
|||
|
11 |
[RuCl2.L11)] |
68 |
4.8 |
7 |
9 |
20 |
|
-68 |
-4.9 |
-7.1 |
-9 |
|||
|
12 |
[RuCl2.L12)] |
68.6 |
4.6 |
9.2 |
11.8 |
18 |
|
-68.8 |
-4.7 |
-9.4 |
-11.9 |
|||
|
13 |
[RuCl2.L13)] |
69.4 |
5 |
9 |
11.2 |
16 |
|
-69.5 |
-5.1 |
-9 |
-11.4 |
|||
|
14 |
[RuCl2.L14)] |
71.2 |
4 |
8.6 |
11 |
14 |
|
-71.3 |
-4.4 |
-8.7 |
-11.1 |
|||
|
15 |
[RuCl2.L15)] |
75 |
4.2 |
8.6 |
11 |
12 |
Table 2: Infrared Spectral data of [RuCl2(L1-15)] Complexes
|
Sr. No |
Complex |
Selected IR bands (cm-1) |
||
|
VC=N |
VRu-N |
V Ru-Cl |
||
| 1 |
[RuCl2.L1)] |
1580 |
520 |
320 |
| 2 |
[RuCl2.L2)] |
1570 |
515 |
310 |
| 3 |
[RuCl2.L3)] |
1575 |
518 |
305 |
|
4 |
[RuCl2.L4)] |
1580 |
515 |
316 |
|
5 |
[RuCl2.L5)] |
1570 |
500 |
300 |
|
6 |
[RuCl2.L6)] |
1580 |
510 |
315 |
|
7 |
[RuCl2.L7)] |
1580 |
505 |
305 |
|
8 |
[RuCl2.L8)] |
1580 |
500 |
320 |
|
9 |
[RuCl2.L9)] |
1600 |
515 |
315 |
|
10 |
[RuCl2.L10)] |
1600 |
518 |
320 |
|
11 |
[RuCl2.L11)] |
1590 |
510 |
320 |
|
12 |
[RuCl2.L12)] |
1580 |
508 |
315 |
|
13 |
[RuCl2.L13)] |
1600 |
515 |
310 |
|
14 |
[RuCl2.L14)] |
1580 |
520 |
305 |
|
15 |
[RuCl2.L15)] |
1580 |
515 |
320 |
Table 3: Ru3p1/2, 3/2; N1s and Cl2p binding energies (eV) in ligand; [RuCl2(L1-15)] complexes.
|
Sr. No |
Ligand & Complexes |
Ru3P1/2, 3/2 |
N1s |
Cl2p |
|
|
Ru3P1/2 |
Ru3P3/2 |
||||
|
1 |
L1 |
— |
— |
400.6 |
— |
|
2 |
L2 |
— |
— |
400.6 |
— |
|
3 |
L3 |
— |
— |
400.6 |
— |
|
4 |
L4 |
— |
— |
400.6 |
— |
|
5 |
L5 |
— |
— |
400.6 |
— |
|
6 |
L6 |
— |
— |
400.6 |
— |
|
7 |
L7 |
— |
— |
400.6 |
— |
|
8 |
L8 |
— |
— |
400.6 |
— |
|
9 |
L9 |
— |
— |
400.6 |
— |
|
10 |
L11 |
— |
— |
400.6 |
— |
|
11 |
L11 |
— |
— |
400.6 |
— |
|
12 |
L12 |
— |
— |
400.6 |
— |
|
13 |
L13 |
— |
— |
400.6 |
— |
|
14 |
L14 |
— |
— |
400.6 |
— |
|
15 |
L15 |
— |
— |
400.6 |
— |
|
16 |
[RuCl2(PPh3)4] |
482.8 |
460.8 |
— |
201.6 |
|
17 |
[RuCl2.L1)] |
482 |
460 |
402.8 |
202.8 |
|
18 |
[RuCl2.L2)] |
482 |
460 |
402.8 |
202.8 |
|
19 |
[RuCl2.L3)] |
482 |
460 |
402.8 |
202.8 |
|
20 |
[RuCl2.L4)] |
482 |
460 |
402.8 |
202.8 |
|
21 |
[RuCl2.L5)] |
482 |
460 |
402.8 |
202.8 |
|
22 |
[RuCl2.L6)] |
482 |
460 |
402.8 |
202.8 |
|
23 |
[RuCl2.L7)] |
482 |
460 |
402.8 |
202.8 |
|
24 |
[RuCl2.L8)] |
482 |
460 |
402.8 |
202.8 |
|
25 |
[RuCl2.L9)] |
482 |
460 |
402.8 |
202.8 |
|
26 |
[RuCl2.L10)] |
482 |
460 |
402.8 |
202.8 |
|
27 |
[RuCl2.L11)] |
482 |
460 |
402.8 |
202.8 |
|
28 |
[RuCl2.L12)] |
482 |
460 |
402.8 |
202.8 |
|
29 |
[RuCl2.L13)] |
482 |
460 |
402.8 |
202.8 |
|
30 |
[RuCl2.L14)] |
482 |
460 |
402.8 |
202.8 |
|
31 |
[RuCl2.L15)] |
482 |
460 |
402.8 |
202.8 |
The binding energies (eV) of prepared ligands i.e. L1-15; [RuCl2(PPh3)4] and [RuCl2(L1-15)] in Table I (Figs.4-6) for Ru3p1/2, 3/2, N1s and Cl2p photoelectron peaks. It was observed that Ru3p1/2, 3/2 binding energies in starting material [RuCl2(PPh3)4] were higher (BE= ~ 482.8 eV and 460.8 eV for Ru3p1/2 and Ru3p3/2) than in prepared [RuCl2L1-15] complexes (Ru3p1/2 BE= 482.0 eV and Ru3P3/2 BE 460.0 eV ); suggesting that electron density in ruthenium metal ion is more in prepared [RuCl2L1-15] complexes than in [RuCl2(PPh3)4] due to coordination [50] (Figs.4-6).
 |
Figure 4: Ru3p1/2 binding energies (eV) in [RuCl2(L1-15)] complexes |
 |
Figure 5: Ru2p3/2 binding energies (eV) in [RuCl2(L1-16)] complexes prepared [RuCl2(L1-15)] metal complexes. |
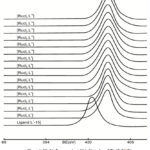 |
Figure 6: N1s binding energies (eV) in Ligands and [RuCl2(L1-15)] complexes prepared. |
The N 1s photoelectron spectra of all these [RuCl2(L1-15)] complexes have shown only one signal symmetrical photoelectron peak towards higher binding energies side ( BE= ~ 402.8 eV) than N1S photoelectron peak of each ligand (~ BE = 400.6 eV) suggesting all four nitrogen atoms of each ligand is coordinated with ruthenium (II) metal ion 50 (Fig.6) & Table.I) The Ru 3s photoelectron peak in all these prepared [RuCl2(L1-15] metal complexes. have shown symmetrical peak at 584.6 i.e. have not shown multiple splitting diamagnetic nature [50]. The Cl2p photoelectron spectra of all these [RuL2(L1-15] complexes have shown one sharp peak at BE~ 202.4-202.0 eV; suggesting inner sphere chlorine atom in trans position 50.
Conclusion
In the present paper, fiffteen Ru(II) complexes of the type [RuCl2L1-15] have been synthesized and characterized with fifteen tetraaza macrocyclic ligands. On account of analytical and spectralstatistics octahedral geometry were provisionallyproposed for all of these complexes.
Acknowledgement
The author AshishRajak is thankful to Chemistry Department, University of Allahabad, Prayagraj for affording financial assistance to the research work and instrumental facilities and Arpit Srivastava, Ramakant and SCS are gratified to UGC-CSIR, New Delhi, India for providing the financial support as UGC JRF (Ref. no- 191620091263), UGC-SRF (Ref. no- 354/CSIR-UGC NET DEC. 2017) and UGC-SRF (Ref. no- 19/06/2016(i)EU-V) respectively.
Conflicts of interest
The authors declareno conflict of interest in the present research work.
References
- Kumar, R.; Singh, R. Russ J Coord Chem.,. 2006, 32(3), 192-198.
CrossRef - Chaudhary, A.; Bansal, N.; Gajraj, A.; Singh, R.V. J inorg biochem., 2003, 96(2-3), 393-400.
CrossRef - Zhong, Z.J.; You, X.Z.; Mak, T.C. Polyhedron., 1994, 13(14), 2157-2161.
CrossRef - Reddy, P.M.; Ho, Y.P; Shanker, K.; Rohini, R. Eur J Med chem., 2009, 44(6), 2621-2625.
CrossRef - Reddy, P.M.; Prasad, A.V; Shanker, K.; Ravinder, V. Spectrochim Acta A Mol Biomol Spectrosc., 2007, 68(3), 1000-1006.
CrossRef - Reddy, P.M.; Prasad, A.V.; Rohini, R.; Ravinder, V. Spectrochim Acta A Mol Biomol Spectrosc., 2008, 70(3), 704-712.
CrossRef - Akine, S.; Sunaga, S.; Taniguchi, T.; Miyazaki, H.; Nabeshima, T. Inorg Chem., 2007, 46(8), 2959-2961.
CrossRef - Anacona, J.R.; Bastardo, E.; Camus, J. Transit Met Chem., 1999, 24(4), 478-480.
CrossRef - Nazmutdinov, R.R.; Roznyatovskaya, N.V.; Glukhov, D.V.; Manyurov, I.; Mazin, V.M.; Tsirlina, G.A.; Probst, M. Inorg Chem., 2008, 47(15), 6659-6673.
CrossRef - Hodacová, J.; Buděšínský, M. Org Lett., 2007, 9(26), 5641-5643.
CrossRef - Constable, E.C.; King, A.C.; Palmer, C.A. Inorganica Chim Acta., 1991, 184(1), 43-45.
CrossRef - Gokel, G.W.; Leevy, W.M.; Weber, M.E. Chem Rev., 2004, 104(5), 2723-2750.
CrossRef - López‐Deber, M.; Bastida de la Calle, R.; Macías, A.; Pérez‐Lourido, P.; Rodríguez, A.; Valencia Matarranz, L. Z Anorg Allg Chem., 2007, 633(11‐12), 1842-1846.
CrossRef - Bértolo, E.; Bastida, R.; De Blas, A.; Fenton, D.E.; Lodeiro, C.; Macías, A.; Rodríguez, A.; Rodríguez-Blas, T. J Incl Phenom Macrocycl Chem., 1999, 35(1), 191-198.
CrossRef - Salavati-Niasari, M.; Davar, F. Inorg Chem Commun., 2006, 9(2), 175-179.
CrossRef - Saleh, A.A. J Coord Chem., 2005, 58(3), 255-270.
CrossRef - Emara, A.A.; Abou-Hussen, A.A. Spectrochim Acta A Mol Biomol Spectrosc., 2006, 64(4), 1010-1024.
CrossRef - Kosmas, C.; Snook, D.; Gooden, C.; Courtenay-Luck, N.S.; McCall, M.J.; Meares, C.F.; Epenetos, A.A. Cancer Res., 1992, 52(4), 904-911.
- Mewis, R.E.; Archibald, S.J. Coord Chem Rev., 2010, 254(15-16), 1686-1712.
CrossRef - Adam, K.R.; Antolovich, M.; Baldwin, D.S.; Duckworth, P.A.; Leong, A.J.; Lindoy, L.F.; McPartlin, M.; Tasker, P.A. J Chem Soc., Dalton Trans., 1993, 1(7), 1013-1017.
CrossRef - El-Boraey, H.A.; El-Gammal, O.A. Spectrochim Acta A Mol Biomol Spectrosc., 2015, 138, 553-62.
CrossRef - Tyagi, M.; Chandra, S.; Akhtar, J.; Chand, D. Spectrochim Acta A Mol Biomol Spectrosc., 2014, 118, 1056-1061.
CrossRef - Bonnett, R. Chem Soc Rev., 1995, 24(1), 19-33.
CrossRef - Champness, N.R.; Frampton, C.S.; Reid, G.; Tocher, D. J Chem Soc., Dalton Trans.,1994, (20), 3031-3037.
- Makki, M.S.; Abdel-Rahman, R.M.; El-Shahawi, M.S. Comptes Rendus Chimie., 2012, 15(7), 617-626.
CrossRef - Canales. J.; Ramirez, J.; Estiu, G.; Costamagna, J. Polyhedron., 2000, 19(22-23), 2373-2381.
CrossRef - Bencini, A.; Bianchi, A.; Giorgi, C.; Paoletti, P.; Valtancoli, B.; Fusi, V.; García-España, E.; Llinares, J.M.; Ramírez, J.A. Inorg Chem., 1996, 35(5), 1114-1120.
CrossRef - Singh, R.V.; Dwivedi, R.; Joshi, S.C. Transit Met Chem., 2004, 29(1), 70-74.
CrossRef - Jain, M.; Gaur, S.; Diwedi, S.C.; Joshi, S.C.; Singh. R.V.; Bansal, A. Phosphorus, Sulfur, and Silicon and the Related Elements., 2004, 179(8), 1517-1537.
CrossRef - Maldonado, C.R.; Salassa, L.; Gomez-Blanco, N.; Mareque-Rivas, J.C. Coord Chem Rev., 2013, 257(19-20), 2668-2688.
CrossRef - Majkowska-Pilip, A.; Bilewicz, A. J Inorg Biochem., 2011, 105(2), 313-320.
CrossRef - El-Boraey, H.A.; El-Din, A.A. Spectrochim Acta A Mol Biomol Spectrosc., 2014, 132, 663-671.
CrossRef - Blain, S.; Appriou, P.; Chaumeil, H.; Handel, H. Analytica Chim Acta., 1990, 232, 331-336.
CrossRef - Chaudhary, A.; Singh, R.V. Phosphorus, Sulfur, and Silicon and the Related Elements., 2007, 182(11), 2647-2665.
CrossRef - Buster, D.C.; Castro, M.M.; Geraldes, C.F.; Malloy, C.R.; Sherry, AD.; Siemers, T.C. Magnetic resonance in medicine., 1990, 15(1), 25-32.
CrossRef - Choi, K.Y.; Chun, K.M.; Suh, I.H. Polyhedron., 2001, 20(1-2), 57-65.
CrossRef - Baker, B.F.; Khalili, H.; Wei, N.; Morrow, J.R.; J Am Chem Soc., 1997, 119(38), 8749-8755.
CrossRef - Buster, D.C.; Gaughan G.T.; Hagan, J.N. U.S. Patent 4885363; 1987.
- Ochiai E. Coord Chem Rev., 2010, 254(15-16), 1812-1814.
CrossRef - Prabhakaran, R.; Geetha, A.; Thilagavathi, M.; Karvembu, R.; Krishnan, V.; Bertagnolli, H.; Natarajan, K. J Inorg biochem., 2004, 98(12), 2131-2140.
CrossRef - Wong, C.Y.; Lee, F.W.; Che, C.M.; Cheng, Y.F.; Phillips, D.L.; Zhu, N. Inorg Chem., 2008, 47(22), 10308-10316.
CrossRef - Shanker, K.; Reddy, P.; Ravinder, V. Int J Chem Tech Res., 2009, 1, 300-307.
- Doro, F.G.; Ferreira, K.Q.; da Rocha, Z.N.; Caramori, G.F., Gomes, A.J.; Tfouni, E. Coord Chem Rev., 2016, 306, 652-677.
CrossRef - Tfouni, E.; Ferreira, K.Q.; Doro, F.G.; da Silva, R.S.; da Rocha, Z.N. Coord Chem Rev., 2005, 249(3-4), 405-418.
CrossRef - Rawling, T.; McDonagh, A. Coord Chem Rev., 2007, 251(9-10), 1128-1157.
CrossRef - Shanker, K.; Reddy, P.; Ravinder, V. Int J Chem Tech Res., 2009, 1, 300-307.
- Jolly, W.L. Coord Chem Rev. 1974, 13(1), 47-81.
CrossRef - Shakir, M.; Nasman, O.S.; Varkey, S.P. Polyhedron., 1996, 15(2), 309-314.
CrossRef - Reddy, P.M.; Prasad, A.V.; Shanker, K.; Ravinder, V. Spectrochim Acta A Mol Biomol Spectrosc., 2007, 68(3), 1000-1006.
CrossRef - Sriastava S. Appl Spectrosc Rev., 1986, 22(4), 401-535.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









