Preparation, Characterization and Analytical Application of Tin (IV) Tungstoselenate - 1, 10 Phenanthroline
Analytical Research Laboratory, Department of Chemistry, Aligarh Muslim University, Aligarh 202 002, India.
Corresponding Author E-mail: ismatanees@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370430
Article Received on : 19-Jul-2021
Article Accepted on :
Article Published : 30 Jul 2021
Reviewed by: Dr. Ahmed Alhusain
Second Review by: Dr. Rosnani Nasution
Final Approval by: Dr.Tasneem mohammad
Synthesis of a composite ion exchange material Tin (IV) tungstoselenate - 1, 10 phenanthroline has been achieved by mixing differentvolume ratios of the organic counterpart with the inorganic ion exchangertin (IV) tungstoselenate. Final sample, having 0.88mmoles of 1, 10 phenanthroline per gram of inorganic ion exchanger, was chosen for characterization, including ion exchange capacity, thermogravimetric analysis, and Fourier transform infrared spectroscopy. The ion exchange capacity of Li+, Na+, Ca2+, Sr2+ metals was determined by using the synthesized material. The adsorption behavior of Al3+,Co2+,Ni2+,Cu2+,Cd2+,Pb2+ in various solvent systems have been studied. Based on distribution Coefficient (Kd) values, few analytically necessary separations of metal ions from the synthetic mixture have been achieved on the column of the composite ion exchanger.
KEYWORDS:Characterization; Composite ion exchanger; Distribution Coefficient; 10 phenanthroline; Tin (IV) tungstoselenate-1
Download this article as:| Copy the following to cite this article: Laiq E, Nabi S. A. Preparation, Characterization and Analytical Application of Tin (IV) Tungstoselenate - 1, 10 Phenanthroline. Orient J Chem 2021;37(4). |
| Copy the following to cite this URL: Laiq E, Nabi S. A. Preparation, Characterization and Analytical Application of Tin (IV) Tungstoselenate - 1, 10 Phenanthroline. Orient J Chem 2021;37(4).Available from: https://bit.ly/3BVGUSW |
Introduction
For decades, inorganic ion exchangers have been known in various fields of sciences due to their radiation and chemical stability, and because of their rigid structures, they possess specific selectivity towards the wide range of metal ions (1). Numerous materials have been synthesized, the most conventional of which were the salts of tetra and pentavalent metals and hydrous oxides. Even the intercalated compounds have become quite interesting because of their varying interlayer space due to the insertion of organic molecules sandwiched between the layers of the inorganic material(2). These intercalated compounds have alternating inorganic and organic layers; as a result, their physical and chemical reactivity can be altered. Derivatized organic-inorganic tetravalent metal acid salts have been developed by the intercalation of polar organic moieties such protonated alcohols, acetones, pyridine, or aromatic compounds within the layers of crystalline α-zirconium phosphate 3-6. They have shown promising features that are important from the analytical point of view.
Several n-alkanols and n-alkylamines have also been intercalated in theγ-Zirconium hydrogen phosphate and γ-titaniumhydrogen phosphate 7. Octa decyltrimethyl ammonium ion was intercalated onto zirconium and titanium dihydrogen phosphate 8.
Organic moieties can also get captured into the pores or adsorbed on the surface of the inorganic matrix to form composite or hybrid ion exchangers. Recently the synthesis of organic-inorganic composite exchange materials has been extensive, as these have the added advantage of inorganic and organic analogs regarding their chemical , thermal stability and ion exchange capacity 9. Composite ion-exchangers have been considered viable in the environmental and analytical chemistry due to their enhanced reproducibility, thermal, chemical, radiational, mechanical stabilities, and selectivity for heavy toxic metals 10. The literature survey revealed that many inorganic ion exchange materials were used to separate metal ions11-12. Inorganic ion exchangers have been extensively used in paper and thin-layer chromatographic separations 13-15. Pollution due to heavy metals ions is a major environmental problem. Industrial effluent contains a massive amount of these toxic heavy metals, the concentration of heavy metals ions in wastewater is up to the maximum level, which is higher than the safe limit; therefore must be removed before they get mixed into the environment. Among the metals, lead, cadmium, copper, aluminium, cobalt are hazardous for living organisms 16 -18.
In this study, an attempt has been made to synthesize an organo-inorganic ion exchanger, Tin (IV) tungstoselenate–1,10phenanthroline. Studies based on its properties, its practical utility have been explored by the selective separation of heavy metals from binary synthetic samples.
Materials and Method
Sodium tungstate dihydrate (BDH, India), Tin (IV) chloride pentahydrate (Baker analyzed, USA), 1,10 Phenanthroline (E.Merck, India),Sodium selenite (BDH, India), and all the other chemicals were of A.R. grade.
Synthesis of inorganic ion exchanger
The inorganic ion exchanger was prepared by mixing the aq. solutions of 0.05M sodium selenite and sodium tungstate with an aqueous solution of 0.05M of tin (IV) chloride (as reported earlier (19 ). The acidic medium was maintained by adding hydrochloric acid to it. The white gelatinous material was obtained, kept in the mother liquor for 12 hours for complete digestion at room temperature. The gel was filtered, washed, dried, and converted to hydrogen form by immersing in 1M HNO3 and was left for 24 hours at room temperature with intermittent stirring. After 24 hours, the supernatant liquid was decanted, and the material was filtered under suction; repeated washing with DMW was done to remove any excess amount of the acid, finally, the obtained product was dried at 40 ± 2ºC.
Derivatization of Tin (IV) tungstoselenate with 1,10 phenanthroline
2.5 grams of the synthesized exchanger in H+ form was reacted with the solution of 1,10 phenanthroline (0.1M) in a temperature-controlled shaker for 6 hrs at 30 ± 2ºC. The pinkish color precipitate was obtained that was filtered, washed, and air-dried. The remaining amount of 1, 10 phenanthroline in the filtrate was titrated with standard hydrochloric acid. The amount of 1, 10 phenanthroline anchored onto tin (IV) tungstoselenate was calculated from the difference between the final and initial concentrations of the 1,10 phenanthroline.
Ion Exchange Capacity
The column was packed with 0.5grams of the derivatized material in hydrogen form. The hydrogen ions were eluted when 0.01M solution of cations was allowed to move through the column of the exchanger. The H+ ions in the effluent were calculated after titrating against the standard NaOH solution. Ion exchange capacity of univalent and bivalent cations are shown in table 1.
Table 1: Ion Exchange Capacity of Tin (IV) tungstoselenate 1,10phenanthroline for different metal ions.
|
Metal ions |
Salt Used |
Ion exchange capacity |
|
Li+ |
LiCl |
0.81 |
|
Na+ |
NaCl |
0.98 |
|
Ca2+ |
Ca(NO3)2 |
1.6 |
|
Sr2+ |
Sr(NO3)2 |
1.4 |
Characterization
The FT-IR was recorded by pressing the derivatized material into the KBr disc using Perkin Elmer 1730 spectrometer. Scanning electron microscopy was done on the ground sample by LEO 435 VP microscope having an imaging device. The thermal behavior of powdered tin (IV) tungstoselenate 1,10 phenanthroline was monitored at a heating rate of 10ºC per minute in a Nitrogen atmosphere using a General V4.IC Du Pont 2100 thermo analyzer. A temperature-controlled shaker from ‘SICO’was used.
Sorption studies
The distribution coefficient (Kd) of metal ions on the Tin (IV) tungstoselenate with 1,10 phenanthroline was determined by the batch method in many solvent systems, 400 mg of the derivatized material in hydrogen form was equilibrated with the solutions of metal ions (40 ml) in the solvent system for about eight hours with occasional shaking. The remaining amount of the cation in solution was determined by titrating against the standard solution of EDTA. The ion-exchange behavior of tin (IV) tungstoselenate 1,10 phenanthroline towards Cu2+, Ni2+, Co2+, Pb2+, Cd2+, Al3+ was studied in different solvents. The Kd values were computed using the formula:
Analytical applications of tin (IV) tungstoselenate 1,10 phenanthroline
Quantitative binary separation of few essential metal ions was attained using the column packed with 2 grams of the synthesized material having nearly 0⋅6 cm internal diameter. An aliquot of the sample (synthetic mixture of metal ions) was transferred in the column of the composite material, and the chosen volume of the elutant was passed through it, keeping a flow rate of around 3-4 drops per minute. EDTA titrations were performed for the quantitative determination of metal ions present in the effluent. Results are shown in Table 3.
Results and Discussion
Based on its chemical studies, the tentative formula of tin (IV) tungstoselenate was given as [(SnO2)7.HSeO3(HWO4) 18].45H2O which was proposed by Nabi et al. (19). As mentioned above, the derivatized product of pink color obtained has 0.88 mmoles per gram of 1,10 phenanthroline anchored on the exchanger. As the result of its chemical composition, the mole ratio of tin (IV) tungstoselenate to 1,10 phenanthroline was assigned as 1.0:5.69. The ion exchange capacity of derivatized material was 1.6 meq per gram, which was quite improved compared with its inorganic counterpart tin (IV) tungstoselenate having 1.4 meq per gram, as Nabi et al. (19).
Scanning electron microscopic photographs of tin (IV) tungstoselenate and tin (IV) tungstoselenate 1,10 phenanthroline at 200x and 200x magnifications are presented in figure 1a and b. Figure 1a reveals that tin (IV) tungstoselenate shows a plate-like morphology, whereas figure 1b indicates a relative change in the structure of the derivatized material. Figure 2 and 3 shows the FTIR spectra of tin tungstoselenate and tin (IV) tungstoselenate 1,10 phenanthroline respectively. In the FTIR spectrum of fig.2 and figure 3, a very broad, intense peak is assigned to the surface hydroxyl stretching band between 3500-2000 cm-1 (15, 20-22). A prominent peak at 1637 cm-1 is due to H-O-H bending (15,20,23,24). The peaks at 1550 cm-1, 1176 cm-1, and 709 cm-1 were assigned to metal-oxygen bonds (22, 25-27). FTIR spectra of tin (IV) tungstoselenate 1,10 phenanthroline shows a small peak at ~800cm-1, a peak at ~700cm-1, and a sharp peak at 475cm-1, respectively, which indicates the presence of tungstate, selenate groups, and metal-oxygen bonds in the material (22,25-27). Metal ligand bond can be predicted in the region below 400 cm-1 (28). The change in intensities of different characteristics peaks indicates the incorporation of organic moiety onto the inorganic matrix.
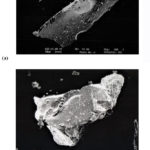 |
Figure 1: SEM images of (a) Tin (IV) tungstoselenate (200x magnification), (b) Tin (IV) tungstoselenate – 1, 10 phenanthroline (200x magnification). |
 |
Figure 2: FTIR spectrum of Tin (IV) tungstoselenate |
 |
Figure 3: FTIR spectrum ofTin (IV) tungstoselenate-1,10 phenanthroline. |
The thermogram of tin (IV) tungstoselenate to 1,10 phenanthroline figure 4 shows definite steps. About 7.5% loss of weight was observed up to 150ºC; this loss of weight corresponds to dehydration of the composite material, characterized by an endothermic peak (figure 5) in the DTA curve. Then weight loss of 10% till 440ºC is due to the evaporation of H2O molecules formed due to the condensation of hydroxyl groups.Additional, 15% weight loss from 440ºC to 520ºC was due to the decomposition of 1,10 phenanthroline, with the evolution of gaseous products, confirmed by an exothermic peak at 490ºC in the DTA curve. Subsequent weight loss starts from 610ºC that continues up to 730ºC, probably due to the vaporization of oxides of selenium. After 730ºC, the weight becomes approximately constant because of the formation of metal oxides. Sorption studies were performed to explore the practical utility of the synthesized material in six different solvent systems. The Kd values are higher for most metal ions studied; data are summarized in table 2. The composite material shows a high affinity towards Cd2+, Pb2+, Al3+and Co2+ in DMW. The distribution coefficients values of Al3+, Co2+, Ni2+, Cu2+ metal ions increases in general when the ratio of nitric acid increases, whereas the decreasing trend is seen for Cd2+ and Pb2+ metal ions. Based on the differential selectivity of metal ions in these solvents systems, some binary separations have been achieved from the synthetic mixtures of metal ions quantitatively by using the column of this composite material. Results summarized in table 3 show that this method can be used for the removal of lead, cadmium, and copper from industrial wastewater.
 |
Figure 4: TGA curve ofTin (IV) tungstoselenate – 1, 10 phenanthroline. |
 |
Figure 5: DTA curve ofTin (IV) tungstoselenate – 1, 10 phenanthroline. |
Table 2: Kd values of metal ions on tin (IV) tungstoselenate – phenanthroline
|
Metal ions |
Solvent System* |
|||||
|
1 |
2 |
3 |
4 |
5 |
6 |
|
|
Al3+ |
5455.55 |
6200 |
9940 |
12350 |
16566.7 |
24900 |
|
Co2+ |
5455.55 |
9980 |
12400 |
16566 |
25000 |
49700 |
|
Ni2+ |
3746.15 |
3776.92 |
7071.42 |
8266.66 |
9940 |
16633.3 |
|
Cu2+ |
4900 |
6175 |
8233.33 |
12400 |
16500 |
24900 |
|
Cd2+ |
26256 |
15068 |
19120 |
18390 |
14150 |
1341 |
|
Pb2+ |
8439 |
7124 |
5498 |
4463 |
3681 |
3001 |
|
*SolventSystem1: DMW, 2: DMSO10% , 3:0.1M HNO3 ,4: 10% DMSO + 0.1M HNO3 1:1 v/v , 5: 10% DMSO + 0.1M HNO3 1:2 v/v ,6 : 10% DMSO + 0.1M HNO3 1:3 v/v |
||||||
Table 3: Quantitative separation of metal ions on the column of tin (IV) tungstoselenate – phenanthroline
|
S. No. |
Separation achieved |
Amount loaded (mg) |
Amount found (mg) |
% Recovery |
Volume of eluent (ml) |
Eluent used |
|
1 |
Ni2+ |
2.90 |
2.81 |
96.89 |
80.00 |
10% DMSO |
|
Co2+ |
2.90 |
2.87 |
98.96 |
90.00 |
DMW |
|
|
2 |
Cd2+ |
5.62 |
5.41 |
96.26 |
60.00 |
10% DMSO + 0.1M HNO3 (1:3 v/v) |
|
Cu2+ |
2.41 |
2.35 |
97.51 |
80.00 |
DMW |
|
|
3 |
Ni2+ |
2.90 |
2.80 |
96.55 |
80.00 |
DMW |
|
Pb2+ |
10.36 |
10.10 |
97.49 |
90.00 |
10% DMSO + 0.1M HNO3 (1:3 v/v) |
|
|
4 |
Cd2+ |
5.62 |
5.44 |
96.79 |
70.00 |
10% DMSO + 0.1M HNO3 (1:3 v/v) |
|
Al3+ |
1.34 |
1.31 |
97.76 |
90.00 |
10% DMSO |
|
|
5 |
Pb2+ |
10.36 |
10.09 |
97.39 |
70.00 |
10% DMSO + 0.1M HNO3 (1:3 v/v) |
|
Cu2+ |
2.41 |
2.35 |
97.51 |
90.00 |
DMW |
Conclusion
A new composite exchange material Tin (IV) tungstoselenate – 1, 10 phenanthroline has been synthesized that exhibits the characteristic features of an ion exchanger having improved ion exchange capacity. Its practicability can further be applied in the field of ion-exchange chromatography, and its analytical significance can be studied in the selective separation and removal of heavy metals from pharmaceutical and industrial wastewater.
Acknowledgement
The authors are thankful to Chairman, Department of Chemistry, Aligarh Muslim University, Aligarh, for providing research facilities.
Conflicts of Interest
There is no conflicts to declare.
References
- Kaushal,S.;Mittal,S.K.;Singh,P. Orient J Chem. 2017,33(4),1726-1735. doi:10.13005/ojc/330417
CrossRef - Schöllhorn,R. Materials and Models: Faces of Intercalation Chemistry. Published online 1994,1-81. doi:10.1007/978-94-011-0890-4_1
CrossRef - Danjo,M.; Baba,Y.;Tsuhako,M.;Nariai,H.;Motooka,I. Phosphorus Research Bulletin.1993,3(0), 25–30. https://doi.org/10.3363/ PRB1992.3.0_25
CrossRef - Maya, L.;Danis,P.O. J.of Chromatog.A.1980,190(1),145-149 doi:10.1016/S0021-9673(00)85523-5
CrossRef - Szirtes, L.;Környei,J.;Pokó, Z. Reactive Polymers, Ion Exchangers, Sorbents.1988.7(2–3), 185–190. https://doi.org/10.1016/0167-6989(88)90138-9
CrossRef - Alberti,G.;Costantino,U. J. of Molecular Catalysis.1984, 27(1–2), 235–250. https://doi.org/10.1016/0304-5102(84)85083-X
CrossRef - Costantino,U. J. Inorg. Nucl. Chem. 1981, 43(8),1895-1902. doi:10.1016/0022-1902(81)80404-6
CrossRef - Ferragina, C.;Cafarelli, P.; Stefanis, A.De.; Rocco,R.Di.;Giannoccaro, P. Materials Research Bulletin.2001,36(10), 1799–1812. https://www.academia.edu/8165480
CrossRef - Pandit,B.;Chudasama,U.M.A. Bull. Mater. Sci.,2001, 24(3), 265-271.10.1007/BF02704920
CrossRef - ALOthman,Z.A.; Inamuddin.; Naushad,M. J. Inorg. Organomet. Polym. Mater. 2013,23(2),257-269. doi:10.1007/s10904-012-9797-2
CrossRef - Rahman,N.; Haseen,U.; Rashid,Arab. J. Chem. 2017,10, S1765-S1773. doi:10.1016/j.arabjc.2013.06.029
CrossRef - Ajiboye, T.O.; Oyewo, O. A.; Onwudiwe, D. C. Chemosphere.2021,262, 128379. https://doi.org/10.1016/J.CHEMOSPHERE.2020.128379
CrossRef - Inamuddin.; Luqman, M. Ion Exchange Technology II: Applications. 2014,Vol 9789400740, 365 doi:10.1007/978-94-007-4026-6
CrossRef - Nabi, S.A.; Laiq, E.; Islam, A. Acta Chromatogr. 2004,14(14),92-101.
- Abdel-Galil, E.A.; Eid, M.A.; Hassan, R.S. Part Sci. Technol. 2020,38(1),113-120. doi:10.1080/02726351.2018.1520764
CrossRef - George, F.; Mahieux, S.; Daniel, C. Microorganisms. 2021,9(2),1-16. doi:10.3390/microorganisms9020456
CrossRef - Bożęcka,A.; Orlof-Naturalna, M.; Sanak-Rydlewska,S. Gospod Surowcami Miner / Miner Resour Manag. 2016,32(4),129-140. doi:10.1515/gospo-2016-0033
CrossRef - Khan, Z.I.; Arshad, N.; Ahmad, K. Environ. Sci. Pollut. Res. Published online 2019,15381-15389. doi:10.1007/s11356-019-04959-9
CrossRef - Siddiqui,Z. A.; Nabi, S. A. Bull.Chem.Soc.Jpn. 1985,58(2), 724-730.
CrossRef - Rao, C. N. R. Chemical applications of infrared spectroscopy. New York: Academic Press)1963,p355.
- Clark, E.N;, Harrison, P.G. J. Organomet. Chem. 1993,463(1-2,85-90. doi:10.1016/0022-328x(93)83402-h)
CrossRef - Davies, M.Infrared spectroscopy and molecular structure. Elsevier, Amsterdam, 1963,p 318
- Abdel-Galil, E. A.;El- Kenany, W. M.; Hussin, L. M. S. Russian Journal of Applied Chemistry,2015. 88 (8),1351–60. doi:10.1134/S1070427215080200
CrossRef - Abdel-Galil, E. A.; Rizk, H. E.; Mostafa, A. Z. Desalination and Water Treatment.2016,57 (38),17880–91. doi:10.1080/ 19443994.2015.1102768
CrossRef - Miller, F.A.; Wilkins, C.H.Anal. Chem.1952,24,1253–1294
CrossRef - Rao, C. N. R. Chemical applications of infrared spectroscopy. New York: Academic Press)1963,p 250
- Gupta, V.K.; Agarwal, S.; Pathania, D.; Kothiyal, N.C.; Sharma, G. Carbohydr. Polym. 2013,96(1),277-283. doi:10.1016/j.carbpol.2013.03.073
CrossRef - Ferraro, J. R.; Basile, L. J.; Kovacic, D. L. Inorganic Chemist. 1966,5(3),392

This work is licensed under a Creative Commons Attribution 4.0 International License.










