Development of a Validated High-Performance Liquid Chromatographic Method in Reverse Phase for Simultaneously Determining Triamcinolone Acetonide and Benzyl Alcohol in Injectable Suspension.
Vivek Tiwari1* and S.K. Mishra2
1Department of Chemistry, Jiwaji University Gwalior (M.P.), 474001, India.
2Department of Chemistry, Government P.G. College, Datia( M.P.)475661 India.
Corresponding Author E-mail: mailme.vivektiwari87@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370423
Article Received on : 22 jun 2021
Article Accepted on : 27 Jul 2021
Article Published : 17 Jul 2021
Reviewed by: Dr. G. M. M. Anwarul Hasan
Second Review by: Dr. Ioana Stanciu
Final Approval by: Dr. Saksit Chanthai
A high-performance liquid chromatographic stability-indicating assaymethod in reverse-phase has been developed and validated for the simultaneous qualitative and quantitative measurement of triamcinolone acetonide and benzyl alcohol content in injectable suspension. The reverse-phase method was developed by using Inertsil ODS-2 column(100 x 4.6mm),5μm. with a mobile phase ratio comprised of water and acetonitrile mixed in 70:30 v/v ratio and pumped at a flow rate of 1.5 ml/min where the temperature of the column oven controlled at 40°C and sample compartment at 5°C with 10μL injection volume. Benzyl Alcohol and Triamcinolone Acetonide had retention times of 1.80 and 5.60 minutes, respectively with UV detection for benzyl alcohol at 215 nm and triamcinolone acetonide at 254 nm. The guidelines of the International Conference on Harmonization were used for the validation of this novel method. During validation, the developed approach was shown to be exact, accurate, linear, robust, rugged, and stable. The detector was showing linear response in a range of 10 µg/mlto 120 µg/ml and 2.5 µg/ml to 30 µg/ml for triamcinolone Acetonide and benzoyl alcohol respectively.
KEYWORDS:Benzyl alcohol; Development; ICH; Stability indicating; Triamcinolone acetonide; Validation
Download this article as:| Copy the following to cite this article: Tiwari V, Mishra S. K. Development of a Validated High-Performance Liquid Chromatographic Method in Reverse Phase for Simultaneously Determining Triamcinolone Acetonide and Benzyl Alcohol in Injectable Suspension. Orient J Chem 2021;37(4). |
| Copy the following to cite this URL: Tiwari V, Mishra S. K. Development of a Validated High-Performance Liquid Chromatographic Method in Reverse Phase for Simultaneously Determining Triamcinolone Acetonide and Benzyl Alcohol in Injectable Suspension. Orient J Chem 2021;37(4). Available from: https://bit.ly/36Fh6Mv |
Introduction
Triamcinolone Acetonide Figure 1 is a synthesized glucocorticosteroid available in salt formhaving immunosuppressive and anti-inflammatory action1. Triamcinolone acetonide interacts with the cytosolic glucocorticoid receptor response element on DNA and affects gene expression. The process of which produces anti-inflammatory proteins and inhibits inflammatory mediators. Therefore, there is a decrease in chronic inflammation and immunological responses1. Triamcinolone acetonide injection is used in treatment for gout, rheumatoid arthritis, synovitis, or osteoarthritis as short term treatment1,and also used to treat atopic dermatitis, contact dermatitis, eczema, psoriasis, lichen planus, lichen sclerosis, subacute cutaneous lupus erythematous with histopathology, and dermatomyositis.2
Triamcinolone Acetonideiupac Name
(1S,2S,4R,8S,9S,11S,12R,13S)-12-fluoro-11-hydroxy-8-(2-hydroxyacetyl) -6,6,9,13-tetramethyl-5,7-dioxapentacyclo [10.8.0.02,9.04,8.013,18]icosa-14,17-dien-16-one.
Structure
 |
Figure 1: Triamcinolone Acetonide Structure |
Molecular formula: C24H31FO6
Molecular weight : 434.50 g/mols
Whereas, Benzyl alcohol Figure 2 is a compound that consists of a benzene ring having a single hydroxymethyl group attached as the substituent which is orthopara directing.It functions as a solvent, metabolite, antioxidant, and perfume.3 In intravenous pharmaceutical formulations, cosmetics, and topical treatments, benzyl alcohol is employed as a preservative at low doses. The USFDA has authorized benzyl alcohol in a 5% solution as safe.3 It’s often utilized in injectable pharmaceutical compositions as a preservative in the concentration range of 0.5–2.0%. The 0.9% saline solution (USP) which is used to flush deposited blood, tissues, or medicationsduring the treatment of patients from dialysis machines, mechanized infusion sets, cannulas, or intravascular catheters after the addition of medicines or the disengagement of blood and the sterile hypotonic distilled water for injection (USP) which is employed for diluting or reconstituting injectable drugs for intravenous use both have concentrations of 0.9 percent benzyl alcohol as a preservative3.
Benzyl Alcoholiupac Name -: phenyl methanol
Structure
 |
Figure 2: Benzyl Alcohol Structure. |
Molecular Formula: C6H5CH2OH
Molecular Weight : 108.14g/mols
The literature review, reveals that various methods of analysis are availablefor triamcinolone acetonide4–7 and benzyl alcohol8–15determination with different formulations or drug matrices, only one methodis available for the tablet or pure form of simultaneous estimation, in which force degradation and recovery studies are performed on standard prepared in diluent instead ofplacebo which does not meet FDA or ICH guidelines criteria to show, specificityor interference due to placebo occurs with the main component16–18and also in previous methods mobile phase is made by mixing a buffer of adjusted pHwith organic to the desired ratio which is a tedious or time-consuming task when analyzing a large number of samples. Buffer in the mobile phase reduces column life and increases the cost of analysis.Still,no method of chromatography is available for direct simultaneous estimation of triamcinolone acetonide and benzyl alcohol in injectable suspension.
This research aims to develop a validated novel, simple, repeatable,linear, specific, high-performance reverse-phase stability-indicating liquid chromatographic method as per ICH guidelines for simultaneouslydetecting triamcinolone acetonide and benzyl alcohol content in injectable suspension in which a force degradationstudy performed on sample andrecovery study on placebo to show drug matrices impact on components of the drugs and buffer is removed from mobile phase preparation to make method simple and cost-effective.
Methods and Materials
Chemical and reagents
AncalimaLifesciences Ltd India provided the triamcinolone acetonide API, benzyl alcohol excipient, and placebo with triamcinolone acetonide and benzyl alcohol for market sample analysis. Ancalima life sciences Ltd.’s market sample Pcort 40mg per 1ml used for sample preparation. Merck limited provided the gradient grade acetonitrile, methanol, and the AR grade, sodium hydroxide, hydrogen peroxide, and hydrochloric acid. The Millipore Milli-Q water purification device was used to create ultra-pure water.
Instrumentation
Equipment and instruments were used in the development and validation experiments as follows: The chromatography was carried out utilizing a water alliance2695 high pressure liquid chromatographic system with 2996 photodiode array detector, degasser, quaternary pump, and autosampler system, as well as Empower3 Software (Waters Corporation, Milford, USA). Thermo lab precision water bath was employed for basic, acidic, and oxidative degradation experiments. In a photostability chamber (Thermolab), a study on photostability was conducted. Thermo hygrometers were used to measure humidity in humidity desiccators. A vacuum oven (Thermolab) was used to conduct the thermal analysis.
Mobile phase and Chromatographic parameters
Table 1: Mobile phase and Chromatographic conditions.
|
Parameters |
Conditions |
|
Column |
Inertsil ODS -2 (100 x 4.60 mm,5μm) |
|
Mobile phase |
Water : Acetonitrile(70:30v/v) |
|
UV detection, nm |
254nm for Triamcinolone Acetonide and 215nm for Benzyl alcohol |
|
Flow rate, mL/min |
1.5 |
|
Injected volume, µl |
10 |
|
ColumnTemperature ,°C |
40 |
Diluent Preparation
Filtered and degassed mobile phase mixed with methyl alcohol in the proportion of 50:50 v\v is used as diluent.
Standard Solutions preparation
Prepared a standard stock solution of benzyl alcohol (1000µg/ml) and Triamcinolone acetonide (4000µg/ml )using diluent, further dilute 5ml of both the stock solution to 250 ml to prepare a standard solution of benzyl alcohol(20µg/ml) and triamcinolone acetonide(80µg/ml).
Sample preparation
Take 2 vials of triamcinolone acetonide injectable suspension, shake well, and transfer contents into 100ml glass volumetric flask with adequate washing of vials from diluent, again added 100ml diluent and sonicate for 2 minutes, use diluent to mark it up to volume and mixed. 5ml of this solution was again diluted to a 50 ml volumetric flask with diluent.
Preparation of placebo solution
Take 2 vials of triamcinolone acetonide injectable suspension, shake well, and transfer contents into 100ml glass volumetric flask with adequate washing of vials from diluent, again added 50ml diluent and sonicate for 2 minutes, use diluent to mark it up to volume and mixed. 5ml of sample solution was again diluted to a 50 ml volumetric flask with diluent.
Forced degradation studies
Acid degradation
Take 2 vials of triamcinolone acetonide injectable suspension, shake well, and transfer contents into 100 ml glass volumetric flask with adequate washing of vials from diluent, again added 50 ml diluent and sonicate for 2 minutes. Further added 5 ml of 1N HCl and mixed well then for 30 minutes, the contents were heated in a hot water bath of thermolab 70°C. Cooled the sample solution and neutralized it with 5ml of 1N NaOH, after neutralization use diluent to mark it up to volume and mixed. 5ml of degraded sample solution was again diluted to a 50 ml volumetric flask with diluent.Figure 3&Figure 4shows the chromatogram obtained.
 |
Figure 3: Acid degradation sample chromatogram at 254nm. |
 |
Figure 4: Acid degradation sample chromatogram at 215nm. |
Alkali degradation
Take 2 vials of triamcinolone acetonide injectable suspension, shake well, and transfer contents into 100 ml glass volumetric flask with adequate washing of vials from diluent, again added 50ml diluent and sonicate for 2 minutes. Further added 5ml of 1N NaOH and mixed well. For 30 minutes, the contents were heated in a hot water bath of thermolab at 70°C. Cooled the sample solution then neutralized it with 5ml of 1N HCl, after neutralization use diluent to mark it up to volume and mixed. 5ml of degraded sample solution was again diluted to a 50 ml volumetric flask with diluent.Figure 5&Figure 6shows the chromatogram obtained.
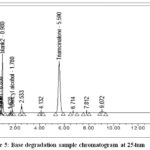 |
Figure 5: Base degradation sample chromatogram at 254nm. |
 |
Figure 6: Base degradation sample chromatogram at 215nm. |
Peroxide degradation
Take 2 vials of triamcinolone acetonide injectable suspension, shake well, and transfer contents into 100 ml glass volumetric flask with adequate washing of vials from diluent, again added 50ml diluent and sonicate for 2 minutes. Further added 5ml of 3.0% H2O2 and mixed well. For 30 minutes, the contents were heated in a hot water bath of thermolab at 70°C. Cool the sample solution up to room temperature then use diluent to mark it up to volume and mix. 5ml of degraded sample solution was again diluted to a 50 ml volumetric flask with diluent. Figure 7 & Figure 8 shows the chromatogram obtained.
 |
Figure 7: Oxidative degradation sample chromatogram at 254nm.. |
 |
Figure 8: Oxidative degradation sample chromatogram at 215nm. |
UV degradation
Take 2 vials of (previously kept in UV light for 24 hrs.)triamcinolone acetonide injectable suspension, shake well, and transfer contents into 100ml volumetric glass flask with adequate washing of vials from diluent, again added 50ml diluent and sonicate for 2 minutes and use diluent to mark it up to volume and mix. 5ml of degraded sample solution was again diluted to a 50 ml volumetric flask with diluent.Figure 9 and Figure 10 shows the chromatogram obtained.
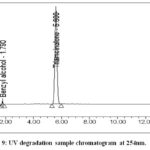 |
Figure 9: UV degradation sample chromatogram at 254nm. |
 |
Figure 10: UV degradation sample chromatogram at 215nm. |
Thermal degradation
Take 2 vials of triamcinolone acetonide injectable suspension, shake well, and transfer contents into 100ml glass volumetric flask with adequate washing of vials from diluent, again added 50ml diluent and sonicate for 2 minutes. For 30 minutes, the contents were heated in a hot water bath ofthermolabat 70°C. Cool the sample solution up to room temperaturethenuse diluent to mark it up to volume and mix. 5 ml of degraded sample solution was again diluted to a 50 ml volumetric flask with diluent.Figure 11 and Figure 12 shows the chromatogram obtained.
 |
Figure 11: Thermal degradation sample chromatogram at 254nm. |
 |
Figure 12: Thermal degradation sample chromatogram at 215nm. |
Method validation
Using International Council for Harmonisation guidelines, the newly designed reverse-phase chromatographic technique was validated for linearity, accuracy, precision, specificity, robustness, ruggedness, the limit of detection, and limit of quantification.16
System suitability
To verify the system’s suitability criteria, before every validation parameter initiation standard preparation injected in five replicates, the system is only suitable for analysis when the % RSD of peak areas and tailing should not be more than 2.0 and theoretical plates should not be less than 2500for triamcinolone acetonide and benzyl alcohol respectively,
Specificity
Establish specificity of the method by demonstrating
Blank, placebo, and as such sample injections will be used to establish that there is no interference from excipients in the solution.
This was demonstrated by forcing the sample to deteriorate with 1N HCl, 1N NaOH, 3.0% H2O2, heated at 70°C for 30 minutes in a hot water bath of thermolab, and exposing for 24 hours in ultraviolet light. According to the test methodology, the samples were prepared. and then injected into a high-performance chromatographic system equipped with water’s 2996 photodiode array detector. The purity angle for the triamcinolone acetonide peak and benzaldehyde peak was less than the threshold value in each case.
Precision
To evaluate system precision, five replicates of the standard preparation were injected into the system as specified in the method of study, and the percent relative standard deviation (RSD) value derived from peak areas was utilized as a measure.For method precision six sample solutions of triamcinolone acetonide injectable suspension prepared as per method and analyzed on the same day. For intermediate precision six prepared sample solutions of triamcinolone acetonide injectable suspension as per method and analyzed using a separate column from the same manufacturer, the different instruments on a different day of method precision. For six preparations, the percent RSD of the assay was determined.
Linearity
Prepared a series of standard preparations of triamcinolone acetonide and benzyl alcohol in the range of 50% to 150% of the target concentration.for triamcinolone acetonide& 12% to 150% of benzyl alcohol. By plotting the absorbance vs. concentration of the drugsthe linearity calibration curves were obtained demonstrating linearity over the concentration range of 10-120 μg/mL for Triamcinolone acetonideand 2.5-30μg/mL for Benzyl alcohol(Table 5 ). MS-Excel was used to compute the slope, intercept, and coefficient of correlation.
The Limit of detection and quantitation
Prepared a series of triamcinolone acetonide and benzyl alcohol standards of different concentrations equal to or below the specified limits using standard stock solutions and injected in triplicate into the HPLC. Limit of detection(LOD) and Limit of quantification (LOQ) was calculated by using the value of slope and intercept of the calibration curves of both the drugs.
Accuracy (Recovery)
The accuracy was carried out by spiking triamcinolone acetonide and benzyl alcohol in the placebo samples at concentrations of 50%, 100, and 150 percent of the target concentration in triplicate (for a total of nine measurements) followed by sample preparation as indicated under the procedure. Mean %assay and %RSD value were calculated for all triplicate preparations.
Robustness
As per the method of analysis,three sample solutionswere prepared of triamcinolone acetonide injectable suspension USP and analyzed using varied chromatographic conditions as below.
Change in flow ( ± 0.2 ml /min.)
Change in column oven temperature (± 2.0)
Change in organic phase composition in the mobile phase (± 5%)
Change in wavelength. (±2 nm)
At each variable condition mean assay percent and %RSD for assay value were calculated along with system suitability criteria for Triamcinolone acetonide and benzyl alcohol.
Solution stability
Solution stability was established by freshly prepared standard and sample keeping it at room temperature and then injecting it freshly and at varied time intervals. % Deviation in the peak area of standard and the sample solution from the initial area was calculated.
Results and Discussion
Method Development Chromatographic parameters optimization
The main purpose of thismethod optimization is to quantitate triamcinolone acetonide and benzyl alcohol content simultaneously, accurately, and precisely without any interference of blank or placebo matrices and for that prepared mobile phase with simple composition without using buffer or adjusting pH. The pKa value for triamcinolone acetonide and benzyl alcohol is 13.4 and 15.4 respectively and both the contents are practically insoluble in water but soluble in methanol hence the approach for development started from the use of water, acetonitrile, and methanol in the mobile phase and diluent preparation. Many columns are used after studying various parameters of development and started with water, acetonitrile ratio (50:50v/v,55:45v/v)with column Hypersil BDS (150 X4.6mm,5μm), at 254nm but the peak of benzyl alcohol elute too early and peak shape was not good hence column changed to Inertsil ODS-2(100X4.6 mm) 5μm with mobile phase ratio water: acetonitrile (50:50) but again peak of benzyl alcohol eluted too early in comparison to triamcinolone acetonide peak and the response of benzyl alcohol peak is not satisfactory hence need to change wavelength for benzyl alcohol peak from 254nm to 215 nm to get a better response from the detector at lower levels.
In a final optimize method the mobile phase ratio of water: acetonitrile (70:30v/v) using column InertsilODS-2(100mmX4.6mm,5μm) thermostated at 40°Cisocratically pumped with a flow rate of 1.5ml/min and 10μl of injection volume.The wavelength for detection of benzyl alcohol and triamcinolone acetonide set at 215nm and 254 nm respectively
Force degradation behavior
Chromatography of triamcinolone acetonide and benzyl alcohol samples under suggested conditions shows degradation behavior
Acid degradation
At 70°C for 30 minutes in 5 mL of 5N hydrochloric acid, moderate deterioration was detected in the sample. (Table 2).
Alkali degradation
In 5 ml of 1N sodium hydroxide solution at 70°C for 10 minutes, the drug was very unstable but excipient benzyl alcohol is stable. (Table 2).
Peroxide degradation
In 5 ml of 3.0 percent H2O2 at 70°C for 30 minutes,the sample was stable. (Table 2).
UV degradation
The sample should be relatively stablewhen exposed in 1.2 million lux hours visible and 200 Watt-hour per square meter UV light. (Table 2).
Thermal degradation
In response to thermal conditions, the sample revealed no substantial deterioration. (Table 2).
Table 2: Force degradation summary of Triamcinolone acetonide (254nm) and Benzyl alcohol(215nm).
|
Conditions and time |
Triamcinolone acetonide at 254nm |
Benzyl alcohol at 215nm |
||||
|
%Assay |
Purity angle |
Purity threshold |
%Assay |
Purity angle |
Purity threshold |
|
|
Sample as is |
99.19 |
0.100 |
0.292 |
100.2 |
0.169 |
0.365 |
|
Acid degradation (5ml 1N HCl, heat 70°C for 30 mins.) |
95.36 |
0.092 |
0.283 |
98.21 |
0.174 |
0.369 |
|
Alkali degradation ( 5ml 1N NaOH, heat 70°C for 10 mins.) |
60.94 |
0.160 |
0.349 |
98.08 |
0.178 |
0.377 |
|
Peroxide degradation (5ml 3.0% H2O2, heat 70°C for 30 mins.) |
99.42 |
0.096 |
0.287 |
99.23 |
0.183 |
0.379 |
|
UV degradation (1.2 million lux hours visible and 200 Watt hour per square meter UV light) |
99.14 |
0.098 |
0.293 |
99.80 |
0.228 |
0.369 |
|
Thermal degradation (heat 70°C, 30 mins.) |
100.61 |
0.094 | 0.288 | 99.20 | 0.211 | 0.388 |
Method Validation
According to International Council for Harmonisation recommendations.16
System suitability
As specified in the method of analysis, system suitability was checked by injecting five replicates of the standard solution on different days of the validation study. The tailing factor, theoretical plates, and peak area %RSD of Triamcinolone acetonide and benzyl alcohol peak from standard solution were computed using the system software The summary of system suitability was found within the acceptance criteria. (See Table 3).
Table 3: Summary of system suitability criteria.
|
Parameters |
Specification |
Observed values |
|||
|
Triamcinolone Acetonide |
Benzyl alcohol |
||||
|
Method precision |
Intermediate precision |
Method precision |
Intermediate precision |
||
|
Area [RSD (%), n = 5] |
≤2.0% |
0.43 |
0.55 |
0.47 |
0.76 |
|
USP tailing |
≤2.0 |
1.05 |
1.11 |
1.19 |
1.31 |
|
Theoretical plates |
NLT 2500 |
6559 |
6249 |
4381 |
4245 |
Precision
For System precision% RSD for replicate injections of triamcinolone acetonide is 0.43 and for benzyl, alcohol is 0.47, respectively (Table 3).
For method precision and intermediate precision %, RSD for assay of triamcinolone is 0.21 and 1.26while for benzyl alcohol is 0.41 and 1.32respectively (Table 4).Overall %RSD for twelve test preparations (six from method precision and six from intermediate precision) (Table 4) of triamcinolone acetonide is 0.90, and for benzyl alcohol is 0.98, which is less than 2.0%. The low RSD value indicates that the technique is precise.
Table 4: Summary of method or intermediate precision.
|
Sample No. |
Triamcinolone Acetonide Assay (% of label claim) |
Benzyl alcohol Assay (% of label claim) |
||
|
Method Precision |
Intermediate Precision |
Method Precision |
Intermediate Precision |
|
|
1 |
99.33 |
100.36 |
100.26 |
100.62 |
|
2 |
99.47 |
99.99 |
100.78 |
100.44 |
|
3 |
99.87 |
98.23 |
101.42 |
98.76 |
|
4 |
99.34 |
101.16 |
100.56 |
101.34 |
|
5 |
99.62 |
101.36 |
101.13 |
101.80 |
|
6 |
99.42 |
98.80 |
100.91 |
98.58 |
|
Mean |
99.51 |
99.98 |
100.84 |
100.26 |
|
SD |
0.21 |
1.26 |
0.41 |
1.32 |
|
%RSD (≤2%) |
0.21 |
1.26 |
0.41 |
1.32 |
|
Overall Mean |
99.75 |
100.55 |
||
|
Overall SD |
0.894 |
0.984 |
||
|
Overall %RSD (≤2%) |
0.90 |
0.98 |
||
Linearity and range
Equations of Linear regression were found to be y = 9431.2x + 5589.6, and y = 17613x + 2358.7, Figure 13 and Figure 14 where as the values of the regression coefficients (r) were 0.9999 for Triamcinolone acetonide and Benzyl alcohol respectively Table 5 shows the proposed method is linear in the given concentration range.
 |
Figure 13: Triamcinolone acetonide Linearity graph. |
 |
Figure 14: Benzyl alcohol Linearity graph. Click here to View figure |
Table 5: Summary of triamcinolone acetonide and benzyl alcohol Linearity.
|
Triamcinolone Acetonide(254nm) |
Benzyl Alcohol(215nm) |
||
|
Concentration (µg/mL) |
Mean Area |
Concentration (µg/mL) |
Mean Area |
|
120.72 |
1141904 |
30.72 |
542507 |
|
100.60 |
956839 |
25.60 |
454226 |
|
80.48 |
768329 |
20.48 |
364924 |
|
60.36 |
569106 |
15.36 |
270251 |
|
40.24 |
385653 |
10.24 |
182731 |
|
20.12 |
198718 |
5.12 |
94189 |
|
10.06 |
98341 |
2.56 |
46530 |
|
Slope |
9431.23 |
Slope |
17613.05 |
|
Intercept |
5589.57 |
Intercept |
2358.71 |
|
Correlation Coefficient(r) |
0.9999 |
Correlation Coefficient(r ) |
0.9999 |
The Limit of detection and quantitation
In this study, the LOD and LOQ values for triamcinolone acetonide were determined to be 0.2012 µg/mL and 0.4020 µg/mL, respectively, while for benzyl alcohol, the values were 0.0205 µg/mL and 0.0512 µg/mL, respectively.
Accuracy (Recovery)
Table 6 shows the mean % assay recovery is within the range of 98.0% –102.0%, Individual assay percentage be in the range of 97.0% to 103.0%, and overall%RSD should not be greater than 2.0. The results show that the recovery of triamcinolone acetonide and benzyl alcohol from the placebo is acceptable.
Table 6: Summary of triamcinolone acetonide(254 nm) and Benzyl alcohol (215nm) recovery.
|
Levels |
Preparation |
Triamcinolone Acetonide at 254nm |
Benzyl alcohol at 215nm |
Limits |
||
|
AmountRecovered % |
% Recovery |
Amount Recovered % |
% Recovery |
|||
|
50% |
1 |
49.02 |
98.04 |
49.52 |
99.03 |
Individual assay 97.0% -103.0% |
|
2 |
49.61 |
99.23 |
49.42 |
98.83 |
||
|
3 |
49.46 |
98.92 |
49.59 |
99.19 |
||
|
100% |
1 |
100.10 |
100.10 |
97.92 |
97.92 |
|
|
2 |
100.38 |
100.38 |
97.24 |
97.24 |
||
|
3 |
99.94 |
99.94 |
97.11 |
97.11 |
||
|
150% |
1 |
149.69 |
99.79 |
147.46 |
98.31 |
|
|
2 |
149.20 |
99.47 |
147.71 |
98.47 |
||
|
3 |
150.00 |
100.00 |
154.15 |
102.77 |
||
|
Mean(98.0%-102.0%) |
99.54 |
Mean |
98.76 |
98.0%-102.0 % |
||
|
Standard Deviation |
0.72 |
SD |
1.67 |
– |
||
|
Overall %Relative Standard Deviation≤ 2.0 |
0.73 |
%RSD≤ 2.0 |
1.69 |
NMT 2.0 % |
||
Robustness
For all the varied chromatographic conditions, the standard solution (theoretical plates, tailingand,%RSD) validity was examined according to system suitability criteria. For each robustness condition, the %assay RSD of Triamcinolone acetonide and benzyl alcohol was computed shown in Table 7&Table 8, the overall %RSD for each set of data should not be more than 2.0.shows that the robustness of the method.
Table 7: Summary of robustness [Triamcinolone acetonide (254nm)].
|
Varied chromatographic parameters |
System suitability parameters observed in the standard |
Triamcinolone Acetonide at 254nm |
||||
|
USP tailing≤ 2.0 |
Theoretical plates |
% RSD≤ 2.0 (n = 5) |
%Assay |
Mean assay% |
%RSD ≤ 2.0 |
|
|
Column temperature (42°C) |
1.05 |
5954 |
0.25
|
98.45 |
98.48 |
0.29
|
|
98.21 |
||||||
|
98.78 |
||||||
|
Column temperature (38°C) |
1.07 |
6857 |
0.19 |
99.99 |
100.03 |
0.20
|
|
99.85 |
||||||
|
100.25 |
||||||
|
Flow rate (1.70 mL/min) |
1.05
|
6963 |
0.98 |
99.61 |
99.88 |
0.32
|
|
100.23 |
||||||
|
99.81 |
||||||
|
Flow rate (1.30 mL/min) |
0.95 |
5700 |
0.55 |
100.91 |
100.98 |
0.29
|
|
100.73 |
||||||
|
101.31 |
||||||
|
Buffer:acetonitrile (664:336) |
1.02 |
6020 |
0.27
|
99.97 |
100.12
|
0.15
|
|
100.12 |
||||||
|
100.28 |
||||||
|
Buffer:acetonitrile (696:304) |
1.04 |
6251 |
0.67 |
101.31 |
100.79 |
0.45
|
|
100.62 |
||||||
|
100.45 |
||||||
|
Wavelength (256 nm) |
1.08 |
6369 |
0.25 |
100.28 |
100.80
|
0.56
|
|
100.71 |
||||||
|
101.4 |
||||||
|
Wavelength (252 nm) |
1.03 |
6757 |
0.37 |
100.84 |
100.95 |
0.31
|
|
100.71 |
||||||
|
101.31 |
||||||
Table 8: Summary of robustness[Benzyl alcohol (215nm)]
|
Varied chromatographic parameters |
System suitability parameters observed in the standard |
Benzyl alcohol at 215nm |
||||
|
USP tailing ≤ 2.0 |
Theoretical plates |
%RSD ≤2.0 (n = 5) |
%Assay |
Mean assay |
%RSD ≤ 2.0 |
|
|
Column temperature (42°C) |
1.19
|
3956 |
0.48
|
99.01 |
99.10 |
0.13
|
|
99.25 |
||||||
|
99.04 |
||||||
|
Column temperature (38°C) |
1.33 |
4755 |
0.80 |
101.11 |
100.78 |
0.83
|
|
99.82 |
||||||
|
101.4 |
||||||
|
Flow rate (1.70 mL/min) |
1.42 |
4876 |
0.77 |
99.33 |
99.75 |
0.57
|
|
99.52 |
||||||
|
100.4 |
||||||
|
Flow rate (1.30 mL/min) |
1.23 |
4054 |
0.57 |
101.32 |
100.59 |
0.94
|
|
99.52 |
||||||
|
100.94 |
||||||
|
Buffer:acetonitrile (664:336) |
1.12
|
4205 |
0.65
|
100.35 |
100.85
|
0.45
|
|
101.25 |
||||||
|
100.94 |
||||||
|
Buffer:acetonitrile (696:304) |
1.15 |
3978 |
0.49 |
101.89 |
101.73 |
0.16
|
|
101.56 |
||||||
|
101.73 |
||||||
|
Wavelength (256 nm) |
1.20 |
4235 |
0.73 |
100.71 |
100.96
|
0.36
|
|
100.78 |
||||||
|
101.38 |
||||||
|
Wavelength (252 nm) |
1.28 |
4412 |
0.52 |
100.88 |
100.91 |
0.32
|
|
101.25 |
||||||
|
100.6 |
||||||
Specificity
By injecting a blank, a placebo, and a sample prepared from the same method, No peaks were observed during the retention time of triamcinolone acetonide and benzyl alcohol peaks, showing that blank and excipient interference is not present.The purity angle of the triamcinolone acetonide peak and benzaldehyde peak was less than the threshold value in each case of force degradation datashown inTable 2, that no degradation products interfered within the retention duration of the triamcinolone acetonide and benzyl alcohol peak.
Stability of solution
Sample and the standard solution are stable for 48 hours at room temperature as the % deviation from the initial area was found to be less than 2.0%. (Table 9)
Table 9: Solution stability of Triamcinolone acetonideand Benzyl alcohol.
|
Time |
Triamcinolone Acetonide(254nm) |
Benzyl Alcohol (215nm) |
||||||
|
Area counts |
% Deviation from initial areaNMT: ±2.0%
|
Area counts
|
Deviation from initial areaNMT: ±2.0%
|
|||||
|
Standard |
Sample |
Standard |
Sample |
Standard |
Sample |
Standard |
Sample |
|
|
Initial |
783472 |
835244 |
0.0 |
0.0 |
363308 |
361941 |
0.0 |
0.0 |
|
3hours |
783924 |
839262 |
0.1 |
0.5 |
364550 |
361479 |
0.3 |
-0.1 |
|
7 hours |
783470 |
849318 |
0.0 |
1.7 |
364001 |
363468 |
0.2 |
0.4 |
|
13 hours |
788259 |
847903 |
0.6 |
1.5 |
365822 |
366594 |
0.7 |
1.3 |
|
22 hours |
790651 |
844587 |
0.9 |
1.1 |
368151 |
364285 |
1.3 |
0.6 |
|
26 hours |
793720 |
847678 |
1.3 |
1.5 |
368900 |
365873 |
1.5 |
1.1 |
|
45 hours |
795720 |
847295 |
1.6 |
1.4 |
368132 |
367300 |
1.3 |
1.5 |
|
48 hours |
798720 |
850596 |
1.9 |
1.8 |
369800 |
368893 |
1.8 |
1.9 |
Conclusion
For the assessment of triamcinolone acetonide and benzyl alcohol content within the presence of sample matrices, placebo, and various degradation conditions an economicalhydrophobic stationary phasestability-indicating high-performance liquid chromatographic method has been created and validated in compliance with the International Council for Harmonisation requirements, which is simple, quick, rugged, accurate, precise and linear. The validation findings of the analytical technique were determined to be adequate. The short runtime of 10 minutes is enough to separate each analyte, and the simple mobile phase composition makes this approach cost-effective and suitable for regular testing of many samples of injections in a short time interval.
Acknowledgment
The author is thankful to Ancalimalifesciences Ltd. for providing placebo of triamcinolone acetonide for analysis of their market sample and also thankful to Elysium pharmaceuticals ltdPadra, Vadodara for providing instruments and analytical support.
Conflict of Interest
The author declares that there is no conflict of interest.
References
- Paik, J.; Duggan, S. T.; Keam, S. J. Drugs2019, 79 (4), 455–462.
CrossRef - Meykadeh, N.; Hengge, U. R. Hautarzt2003, 54 (7), 641–661.
CrossRef - Johnson, W.; Bergfeld, W. F.; Belsito, D. V.; Hill, R. A.; Klaassen, C. D.; Liebler, D. C.; Marks, J. G.; Shank, R. C.; Slaga, T. J.; Snyder, P. W.; Andersen, F. A. Int. J. Toxicol.2017, 36 (3_suppl), 5S-30S.
CrossRef - Döppenschmitt, S. A.; Scheidel, B.; Harrison, F.; Surmann, J. P. J. Chromatogr. B Biomed. Appl.1996, 682 (1), 79–88.
CrossRef - van Heugten, A. J. P.; de Boer, W.; de Vries, W. S.; Markesteijn, C. M. A.; Vromans, H. J. Pharm. Biomed. Anal.2018, 149, 265–270.
CrossRef - Kedor-Hackmann, E. R. M.; Gianotto, E. A. S.; Santoro, M. I. R. M. Anal. Lett.1997, 30 (10), 1861–1871.
CrossRef - Muralidharan, S.; Venugopal, V.; Kumar, J.; Parasuraman, S. Turkish J. Pharm. Sci.2016, 13 (1), 9–16.
CrossRef - Čudina, O. A.; Čomor, M. I.; Janković, I. A. Chromatographia2005, 61 (7–8), 415–418.
CrossRef - Ghasemi, J.; Niazi, A.; Ghobadi, S. Pharm. Chem. J.2005, 39 (12), 671–675.
CrossRef - Di Pietra, A. M.; Cavrini, V.; Raggi, M. A. Int. J. Pharm.1987, 35 (1–2), 13–20.
CrossRef - Tan, H. S. I.; Manning, M. A.; Hahn, M. K.; Tan, H. G. H.; Kotagal, U. R. J. Chromatogr. B Biomed. Sci. Appl.1991, 568 (1), 145–155.
CrossRef - Rizk, M.; Ibrahim, F.; Hefnawy, M.; Nasr, J. J. Acta Pharm.2007, 57 (2), 231–239.
CrossRef - El Sherbiny, D.; Wahba, M. E. K. J. Taibah Univ. Sci.2020, 14 (1), 294–304.
CrossRef - Hewala, I.; El-Fatatre, H.; Emam, E.; Mubrouk, M. Talanta2010, 82 (1), 184–195.
CrossRef - Khade, V.; Mirgane, S. Int. J. Sci. Eng. Res.2014, 5 (11), 887–889.
- ICH Guideline, Q2 (R1), Validation of Analytical Procedures: Text and Methodology, 2005.available fromhttps://database.ich. org/sites/ default/files.
- World Health Organization; Guidelines on validation – appendix 4 Analytical method validation, 2018.available fromhttps:// www.who. int/medicines/areas/quality_safety/quality_assurance/28092018Guideline_Validation_AnalyticalMethodValidation-Appendix4_QAS1. .
- ICH guideline, Q1A (R2): Stability Testing of New Drug Substances and Products, International Conference on Harmonization, Geneva. (https://www.ich.org/page/quality-guidelines), 2003.
- Blessy, M.; Patel, R. D.; Prajapati, P. N.; Agrawal, Y. K. J. Pharm. Anal.2014, 4 (3), 159–165.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









