Synthesis and Properties of Ce3+ Doped the Multi-Functional Tio2-Sio2 nanocomposite Films
Tran Thi Quynh Nhu1*, Tran Trong An1, Cao Xuan Thang1* , Nguyen Viet Tung2, Nguyen Thanh Phuong2 and Lai Trung Tung3
, Nguyen Viet Tung2, Nguyen Thanh Phuong2 and Lai Trung Tung3
1Advanced Institute for Science and Technology (AIST), Hanoi University of Science and Technology (HUST), No 01, Dai Co Viet Road, Hanoi, Vietnam
2Institute of Science and Technology, Ministry of Public Security, No 100, Chien Thang Road, Hanoi, Vietnam.
3R and D center of Kangaroo Groups, No 05, Dao Duy Anh Road, Hanoi, Vietnam
Corresponding Author E-mail: thang.caoxuan@hust.edu.vn
DOI : http://dx.doi.org/10.13005/ojc/370307
Article Received on : 29-Apr-2021
Article Accepted on :
Article Published : 03 Jun 2021
The effect of Cerium on the properties of the multi-functional SiO2-TiO2:Ce3+ nanocomposite films are shown in this paper. The multi-functional SiO2-TiO2:Ce3+ nanocomposite film was synthesis by a sol-gel method and coated several layers on a glass substrate by a spin-coating technique. The bonding was indicated in the obtained film that Si-O-Si and Si-O-Ti bonding, which are characteristics that bonding in the SiO2-TiO2 matrix by the FT-IR spectrum. The TiO2 crystal in the anatase phase and SiO2 in the amorphous phase of the nanocomposite film was determined by X-ray Diffraction. When increased doping of Ce3+, the prepared films have a high transmittance in the visible light (from 84.5 to 88.3%, 400 – 800 nm), the red-shifted of the absorbance edge in UV(A) region (from 355 to 390 nm), and the optical bandgap is narrowed. Particularly, the SiO2-TiO2:6%Ce3+ film has the transmittance by 88.3% in the visible light (400 – 800 nm), absorbance in the UV(A) light (390 nm), and performance the super-hydrophilic with/without thermal and UV(A) light. Thus, the multi-functional SiO2-TiO2:6%Ce3+ nanocomposite film could have a potential to use as the protective high transmittance film to avoid the UV-A light and exhibits a super-hydrophilic on the material surface such as glasses, photovoltaic devices, window panels.
KEYWORDS:Ce3+ Doped Tio2/Sio2; Sol-Gel; Tio2/Sio2 Thin Films; Tio2/Sio2nanocomposite
Download this article as:| Copy the following to cite this article: Nhu T. T. Q, An T. T, Thang C. X, Tung N. V, Phuong N. T, Tung L. T. Synthesis and Properties of Ce3+ Doped the Multi-Functional Tio2-Sio2nanocomposite Films. Orient J Chem 2021;37(3). |
| Copy the following to cite this URL: Nhu T. T. Q, An T. T, Thang C. X, Tung N. V, Phuong N. T, Tung L. T. Synthesis and Properties of Ce3+ Doped the Multi-Functional Tio2-Sio2nanocomposite Films. Orient J Chem 2021;37(3). Available from: https://bit.ly/3g4dlUX |
Introduction
Materials used outdoors are often damaged by ultraviolet rays and extreme weather conditions or by mechanical collisions with other materials. In recent years, the development of nano-sized coatings that both ensure aesthetics and protect surfaces in extreme conditions is of particular concern. The most prominent is the thin film synthesized from TiO2 material, which has been developed for a long time but is still current because of their application properties in the protective film field. Titanium dioxide is a semiconductor oxide with a wide band gap, located in the ultraviolet region (3.0 – 3.2 eV) [1]. TiO2 has attracted a lot of research scientists because it has interesting properties such as TiO2 in Anatase phase with photocatalytic activity, self-cleaning ability, some studies show that TiO2 materials are also capable of antibacterial 1-3. However, Anatase phase has photocatalytic activity, under the effect of UV rays, can decompose organic components in artificial rocks, damaging the rock surface. In addition, Anatase phase is not stable to heat, when the heating temperature increases, TiO2 materials have the ratio of Anatase and Rutile phases changes in the direction of increasing the Rutile phase ratio 4. Moreover, TiO2 has poor mechanical strength and ability to adhere to the surface of other materials.
As an alternative to TiO2, silica-titania composite material, which is an oxide system consisting of silica (SiO2) and titania (TiO2), can be used. This material system has been the subject of research by many scientists because of the unique properties that come from the combination of these two separate oxides.
The silica-titania composite exhibits many desirable properties such as ultraviolet light absorption, TiO2 self-cleaning, antimicrobial material, both the stability, mechanical strength of SiO2, and incoming properties from chemical bonding between two materials 5-9. In addition, silica and titania are both environmentally friendly, non-toxic, and low cost materials 2.
To enhance absorption and to improve some properties, materials can be doped with certain rare earth metals, transitional or nonmetallic. Cerium is a rare earth metal known for its many redox states. As an important doping material, cerium has advantages such as multiple electronic energy levels and valence states, which lead to different properties 10,11. When doping cerium into the silica-titania composite material, there is a shift in the absorbed light from the ultraviolet to the visible light, enhancing the photocatalytic activity 11.z
According to our knowledge, up to now, there has been no published study on the properties ofCe-doped TiO2/SiO2nanocomposite thin film and orientation for application in the field of coating for outdoor use. In this study, we have proposed the successful synthesis of Ce-doped TiO2/SiO2nanocomposite thin film by the sol gel method combined using the spin coating technique and their properties are discussed details
Experimental
Materials
Tetraethyl orthosilicate (TEOS, 99% Sigma-Aldrich), Titanium tetra-n-butoxide (TBT, 98%, Sigma-Aldrich), Cerium (III) nitrate hexahydrate (Xilong Scientific) were used as starting materials. Isopropanol (IPA, 99.7%, Xilong Scientific), diethylene glycol (DEG, 99.7%, Xilong Scientific) were used as a solvent. The molar ratio of TEOS and TBTwas optimized and fixed at 70:30.
Preparation
A series of TiO2-SiO2:xCe3+ nanocomposite films (x = 0 ÷ 0.08 mol) were prepared by the pechini-type sol–gel method and spin-coating technique. In the typical synthesis process, 7 mmol (1,55 mL) of TEOS was mixed with 25 mL IPA stirred for 10 – 15 minutes by a magnetic stirring hot plate at room temperature. Then HNO3 and water were added into the solution, followed by stirring for 1 hour. Then, 3 mmol (1mL) Titanium n-butoxide (TBT) was added into the solution which was further stirred for 1 hour. Thirdly, molar ratios of ([Ce3+]/([Ti4+] + [Si4+]) with 2, 4, 6, and 8% of Ce(NO3)3.6H2O were alternatively added into the solution, then continuously stirred for 30 minutes. Finally, DEG (1 mL) was prepended as a cross linking agent and the stirring continued for 15 minutes to get a complete homogeneous solution. To prepared films form, they were spin- coated onto a glass substrate by a spin-coater machine, following two stages: 1500 rpm for 7s and 2500 rpm for 30s. Each of five nanocomposite layers on the glass substrate was dried at several times to evaporate solvents. After that, the five-layer films were annealed in the air at 100, 200, 300 oC for 2 hours. The obtained nanocomposite films were employed to assess the further characterization.
Characterization
After obtaining the nanocomposite material, we carried out measurements to characterize and analyze the properties of the material. In detail, the Fourier- transform infrared spectroscopy (FT-IR, Spectrum Two, Perkin Elmer) was used to study the chemical bonding in the nanocomposite material. The crystalline structure of nanocomposite films was studied by X-ray Diffraction (D8-ADVANCE, Bruker-Germany). Thesurface morphology of films was characterized by the high-resolution field emission scanning electron microscopy (FE-SEM, JEOL JSM-7600F). The optical property of the films was investigated by using UV-vis spectrophotometer (JASCO V-750). The wettability behavior was studied by the water contact angle measurement (WCA, C017).
Results and discussion
Fig.1. shows the XRD patterns of SiO2-TiO2:6 %molCe3+ nanocomposite thin film on the glass substrate air-annealed at 100, 200 and 300 oC for 2 hours (a,b,c samples). The XRD patterns of (a,b) samples show that the films consist of SiO2and TiO2in the amorphous phase but at 300 oC, the sample also existed anatase phase which is located at 2θ = 23o and TiO2 crystals in the anatase crystal due to the characteristic peak at 2θ = 55.9o; 31.7o; 66.9o (PDF#84-1286). It is noteworthy that not all the peaks of the anatase phase and no peak of Ce crystal phase are observed in the XRD patterns. Such observed can be attributed to thickness of thin-film and the greater ionic radii of Ce3+ (0.107 nm) than Ti4+, Si4+ (0.068, 0.040 nm, respectively). The difference in radii leads to difficulty for Ce3+ ions to replace Ti4+ or Si4+ ions in the SiO2-TiO2 matrix, hence, Ce3+ ions tend to bond with O2- anion on the surface of TiO2[12]. The trendy of bonding of Ce3+ with O2- on its surface is a factor to influence the optical and wetting properties of the nanocomposite film that would be discussed in the next section.
 |
Figure 1: X-Ray of Ce3+ doped TiO2/SiO2 with 6% mol concentration at different temperatures for 2 hours (a:100 oC; b: 200 oC; c: 300 oC) |
The FT-IR spectra of SiO2-TiO2 and SiO2-TiO2:6%Ce3+nano composite thin film (air-annealed at 300 o for 2 hours) shown in Fig 2. The bands at 1070 cm-1 and 791 cm-1 can be assigned to the vibration of Si-O-Si bonding in SiO2 and This trend indicated that vibration at 901 cm-1 maybe resulted from the Ti–O–Si linkagesSi-O-Ti bonding in TiO2-SiO213, 14. In this case, the content of Ce3+ is not sufficient to influence the vibration of bonding in the SiO2-TiO2 matrix.
 |
Figure 2: FT-IR spectrum TiO2/SiO2 (a); TiO2/SiO2: 6%mol Ce3+at 300 oC for 2 hours. |
The morphology of SiO2-TiO2:x%Ce3+nanocomposite films with a molar different ratio of Ce3+ (x = 0, 2, 4, 6, 8%) air-annealed at 300 oC for 2 hours is captured in the FE-SEM images (Fig.3). As seen in FE-SEM images, the morphology of SiO2-TiO2 nanocomposite films change with respect to the amount of Ce3+ ions doped in the film. It is clearly seen, the composite film with x = 0% shows some cracks on its surface, however, these cracks disappear when increasing Ce3+ ratio to 2% (Fig.3b). In addition, the surface of SiO2-TiO2:x%Ce3+ composite films display the hole-like nanoparticles that distribute in the matrix with (x = 4%, 6%) (Fig3. c, d) and the hole-like particles are distributed in the matrix having an average size of 50 – 100 nm.Further increasing the ratio of Ce3+ (x = 8%) in Fig.3e, the cracks re-appear in the SiO2-TiO2:Ce3+ composite film surface. The appearance of particles on the nanocomposite film surface could be attributed to high enough concentration of Ce3+ doped in the film to induce phrase separation and form particles on the film surface.
 |
Figure 3: FE-SEM images of Ce3+-doped SiO2-TiO2 nanocomposite thin film at 300 oC in the air for 2 hours with a molar different ratio of Ce3+ (a) 0% (b) 2% (c) 4% (d) 6% (e) 8% (f) distribution of particles size of 6% Ce3+ sample |
Fig.4 shows the UV-vis transmittance spectrum of the obtained nanocomposite films. For the application of self-cleaning and protection, the film has to meet requirements of transmittance that greater than 85% in the visible region (400 – 800 nm), and absorbance in the UV-A region (300 – 400 nm) 8. It is clear to observe that the concentration of Ce3+ ion-doped in the prepared film is a factor to effect to its optical properties. As shown in Fig.4a, all of the wavelengths in the visible region (400 – 800 nm) can be transmitted through the film. By doping Ce3+, the transmittance (T) of the SiO2-TiO2 film sample is raised from 84.5% to 88.3%. Because the intrinsic bandgap of SiO2-TiO2 composite is 3.0 – 3.4 eV 15. In addition, the morphology of the Ce3+-doped film surface shows that particles are distributed relatively with at least optical defects, due to low light scattering of the films, so then the transmittance of film increased. For all of those reasons, the visible light is easily transmitted in the nanocomposite film 15, 16.
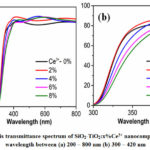 |
Figure 4: UV-vis transmittance spectrum of SiO2-TiO2:x%Ce3+ nanocomposite films with wavelength between (a) 200 – 800 nm (b) 300 – 420 nm. |
On the other hand, as can be seen in Fig.4b, the absorbance is shifted towards longer wavelength from 300 – 420 nm to 355 to 390 nm, when the molar proportion of Ce3+ ions increases. The red-shifted of the absorbance can evidence for Ce3+ successfully doped in the SiO2-TiO2 matrix. However, with the Ce3+ concentration is 8%, the change is not significant in the UV-vis spectrum, also in the FE-SEM image that could be the concentration of Ce3+ is saturated in the SiO2-TiO2 matrix. Consequently, the molar ratio of Ce3+ doped in the film is optimized at 6%.
To investigate the effect of Ce3+ on the bandgap of the SiO2-TiO2nanocomposite, the samples prepared and annealed at 300 oC for 2 hours. Fig.5 illustrates the UV-vis Diffuse Reflection Spectrum (UV-vis DRS) of the SiO2-TiO2:x%Ce3+. In Fig.5a, it can be seen that the absorption edge of the Ce3+-doped in the samples is shifted towards the red-wavelengths when the Ce3+ concentration increase (from 390 to 530 nm). It is well known that the dopant can influence the structure of the host material. The configuration of Ce3+ is [Xe]4f15d06s0 of which its f-orbital has unoccupied. When Ce3+ was doped in the SiO2-TiO2 matrix, the 4f electronic configuration of Ce which allows electron transition from 2p levels of oxygen (valance band – VB) to 4f levels of Cerium and 2p levels of Oxygen to 3d levels of Titanium (conduction band – CB) 17-21. This leads to the generation of electron-hole pairs and the improvement of the visible light absorption, and visible light response.
According to the theory of P.Kulbelka and Munk 22 presented in 1931, the optical bandgap is calculated by applying the Kubelka-Munk function
(1(F(R∞)hν)1/γ = B(hν – Eg) (1)
Where R∞ is the reflectance of an infinitely thick specimen, h is the Planck constant, ν is the photo’s frequency, Eg is the bandgap energy, B is a constant. The γ factor depends on the nature of the electron transition and is equal to ½ or 2 for the direct and indirect transition band gaps, respectively. It is known that SiO2, TiO2 are direct transition bandgap, therefore, Eg can be determined by plotting (F(R∞)hν)2 versus hν the optical bandgap 23.
 |
Figure 5: UV-vis Diffuse Reflection Spectrum of SiO2-TiO2:x% Ce3+ nanocomposite films (a) the UV-vis absorbance spectrum (b) and the calculated band gap by Tauc plot. |
The optical bandgap of all samples is calculated and shown in Fig.5b, for the SiO2-TiO2nanocomposite sample, the optical bandgap is 3.68 eV, with absorbance wavelength is 337 nm, respectively. With an increase of molar ratio of Ce3+ doped in the SiO2-TiO2 matrix, the optical bandgap of obtained sample declines from 3.4 to 2.95 eV. When Ce3+ doped in the SiO2-TiO2 matrix, the 4f levels of Cerium are occupied, leads to new electric states in the bandgap of TiO2, which were reported by Density-functional theory (DFT) simulation articles. According to DFT simulation for Ce3+ doped in the TiO2, the 4f-Ce states are near conduction bands, they are shallow impurity states. This leads to the red-shifted of the absorption edge and the bandgap of the prepared nanocomposite material is narrowed. In addition, the shallow impurity levels are a factor to influenced the generation of photo-excited electron-hole pairs because they can trap the photo-excited electrons and holes 24. Therefore, when Ce3+ is doped in the SiO2-TiO2 matrix, the optical properties of the SiO2-TiO2 nanocomposite is influenced.
 |
Figure 6: The water contact angle of the SiO2-TiO2:6%Ce3+nanocomposite film was exposed UV radiation 30 minutes (a: non-coated; b: coated by SiO2-TiO2:6%Ce3+) |
The wettability of the nanocomposite films is investigated by the contact angle method (WCA). To further investigate the surfaces of the Cedoped SiO2-TiO2 nanocomposite films, the wettability behavior of the films was investigated by the water contact angle (WCA) method. Fig. 6 shows the water contact angle measurements for the non-coated sample and the sample coated with a layer of 6 mol% Ce-doped SiO2-TiO2 film after being exposed to UV (365 nm) for 30 min. While the WCA of the noncoated sample did not change, the angle of the coated film changes from 43o to 11o. This result indicates that the surface of the sample has been transformed from a hydrophilic surface to asuper-hydrophilic surface when coating with 6 mol% Ce-doped SiO2-TiO2 film. From the literatures, this transformation process can be explained by the creation of surface oxygen vacancies due to the appearance of TiO2nanocrystals in the coated film. In such system, the water molecules may travel into the oxygen vacancy sites, leading to the dissociative adsorption of the water molecules on the surfaces of coated film and the photoinduced reconstruction of surface hydroxyl groups. With the increase of adsorbed hydroxyl groups on the film surface, van der Waals forces and hydrogen bond interactions between water molecules and hydroxyl group will increase, leading to the super-hydrophilic properties of the composite film 25.
Conclusion
In conclusion, the multi-functional SiO2-TiO2:x%molCe3+ nanocomposite film was successfully synthesized by sol-gel method. The results reported in this study illustrate that the Ce3+ ion is a factor to influence the morphology, optical property, and the wetting of the obtained film. Particularly, the multi-functional SiO2-TiO2:6%molCe3+ nanocomposite film is a highly transmittance in the visible light (greater than 85%), absorbance in UV(A) light, and super-hydrophilic surface. For all of those results, the SiO2-TiO2:6%molCe3+ film is recommended to use as the protective film with some surfaces like glasses, photovoltaic devices.
Acknowledgement
This research is funded by the Hanoi University of Science and Technology (HUST) underproject number T2020-SAHEP-032. The author thankfulto R&D center of Kangaroo groups for their kind support and motivation.
Conflict of interest
No Conflict of interest.
References
- N. Mufti, I.K.K. Laila, Hartatiek, A. Fuad, J. Phys. Conf. Ser., 853(1), (2017).
CrossRef - N. Rahimi, R.A. Pax, E.M.A. Gray, Prog. Solid State Chem., 44(3), 86-105 (2016).
CrossRef - Y. Hendrix, A. Lazaro, Q. Yu, J. Brouwers, World J. Nano Sci. Eng., 05(04), 161-177 (2015).
CrossRef - Jaroenworaluck, A., N. Pijarn, N. Kosachan, and R. Stevens, “Nanocomposite TiO2-SiO2 gel for UV absorption,” Chem. Eng. J., vol. 181–182, pp. 45–55, 2012.
CrossRef - Erdural, B., U. Bolukbasi, and G. Karakas, “Photocatalytic antibacterial activity of TiO2-SiO2 thin films: The effect of composition on cell adhesion and antibacterial activity,” J. Photochem. Photobiol.A Chem., vol. 283, pp. 29–37, 2014.
CrossRef - Jesus, M. A. M. L. de, J. T. da S. Neto, G. Timò, P. R. P. Paiva, M. S. S. Dantas, and A. de M. Ferreira, “Superhydrophilic self-cleaning surfaces based on TiO2 and TiO2/SiO2 composite films for photovoltaic module cover glass,” Appl. Adhes. Sci., vol. 3, no. 1, pp. 1–9, 2015.
CrossRef - Sun, S., T. Deng, H. Ding, Y. Chen, and W. Chen, “Preparation of nano-TiO2-coated SiO2 microsphere composite material and evaluation of its self-cleaning property,” Nanomaterials, vol. 7, no. 11, 2017.
CrossRef - Watanabe, F., “Composite resin.,” Nihon ShikaIshikaiZasshi, vol. 24, no. 5, p. 483, 1971.
- Zhang, S., D. Sun, Y. Fu, and H. Du, “Recent advances of superhardnanocomposite coatings: A review,” Surf. Coatings Technol., vol. 167, no. 2–3, pp. 113–119, 2003.
CrossRef - Vázquez-Velázquez, A. R., M. A. Velasco-Soto, S. A. Pérez-García, and L. Licea-Jiménez, “Functionalization effect on polymer nanocomposite coatings based on TiO2–SiO2 nanoparticles with superhydrophilic properties,” Nanomaterials, vol. 8, no. 6, 2018.
CrossRef - Zayat, M., P. Garcia-Parejo, and D. Levy, “Preventing UV-light damage of light sensitive materials using a highly protective UV-absorbing coating,” Chem. Soc. Rev., vol. 36, no. 8, pp. 1270–1281, 2007.
CrossRef - Maon, L., (2013), “Mohs’ Scale of Hardness” J. Chem. Inf. Model., vol. 53, no. 9, pp. 1689–1699, 2013.
CrossRef - Latthe S, Liu S, Terashima C, Nakata K, Fujishima A (2014) Transparent, Adherent, and Photocatalytic SiO2-TiO2 Coatings on Polycarbonate for Self-Cleaning Applications. Coatings 4:497–507. doi: 10.3390/coatings4030497.
CrossRef - Sun X, Li C, Ruan L, Peng Z, Zhang J, Zhao J, li Y (2014) Ce-doped SiO2@TiO2 nanocomposite as an effective visible light photocatalyst. J Alloys Compd 585:800–804. doi: 10.1016/j.jallcom.2013.10.034
CrossRef - Latthe S, Liu S, Terashima C, Nakata K, Fujishima A (2014) Transparent, Adherent, and Photocatalytic SiO2-TiO2 Coatings on Polycarbonate for Self-Cleaning Applications. Coatings 4:497–507. doi: 10.3390/coatings4030497
CrossRef - Guan K (2005) Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf Coatings Technol 191:155–160. doi: 10.1016/j.surfcoat.2004.02.022
CrossRef - Jiang Y, Jin Z, Chen C, Duan W, Liu B, Chen X, Yang F, Guo J (2017) Cerium-doped mesoporous-assembled SiO2/P25 nanocomposites with innovative visible-light sensitivity for the photocatalytic degradation of organic dyes. RSC Adv 7:12856–12870. doi: 10.1039/c7ra00191f
CrossRef - Cao Y, Zhao Z, Yi J, Ma C, Zhou D, Wang R, Li C, Qiu J (2013) Luminescence properties of Sm3+-doped TiO2 nanoparticles: Synthesis, characterization, and mechanism. J Alloys Compd 554:12–20. doi: 10.1016/j.jallcom.2012.11.149
CrossRef - Devi LG, Kumar SG (2012) Exploring the critical dependence of adsorption of various dyes on the degradation rate using Ln 3+ -TiO 2 surface under UV/solar light. Appl Surf Sci 261:137–146. doi: 10.1016/j.apsusc.2012.07.121
CrossRef - Tsega M, Dejene FB (2016) Structural and optical properties of Ce-doped TiO2 nanoparticles using the sol-gel process. ECS J Solid State Sci Technol 5:R17–R20. doi: 10.1149/2.0341602jss
CrossRef - Yao Y, Zhao N, Feng JJ, Yao MM, Li F (2013) Photocatalytic activities of Ce or Co doped nanocrystalline TiO 2-SiO2 composite films. Ceram Int 39:4735–4738. doi: 10.1016/j.ceramint.2012.11.035
CrossRef - Džimbeg-Malčić V, Barbarić-Mikočević Ž, Itrić K (2012) kubelka-munk theory in describing optical properties of paper (II). Teh Vjesn 19:191–196
- Makuła P, Pacia M, Macyk W (2018) How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J Phys Chem Lett 9:6814–6817. doi: 10.1021/acs.jpclett.8b02892
CrossRef - Xie K, Jia Q, Wang Y, Zhang W, Xu J (2018) The electronic structure and optical properties of Anatase TiO2 with rare earth metal dopants from first-principles calculations. Materials (Basel) 11. doi: 10.3390/ma11020179.
CrossRef - Vidyadharan V, Vasudevan P, Karthika S, Joseph C, Unnikrishnan N V., Biju PR (2015) Structural, Optical and AC Electrical Properties of Ce3+-Doped TiO2–SiO2 Matrices. J Electron Mater 44:2754–2761. doi: 10.1007/s11664-015-3724-6
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










0 Comments