Kinetics and Mechanism of Oxidation of Aliphatic Secondary Alcohols by Benzimidazolium Fluorochromate in Dimethyl Sulphoxide Solvent
Department of Chemistry, J N V University, Jodhpur 342 001, India
Corresponding Author E-mail: pallavianuk@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370315
Article Received on : 13-Mar-2021
Article Accepted on :
Article Published : 25 Jun 2021
Oxidation of secondary alcohols is an important part of synthetic organic chemistry. Various studies are carried out at different reaction conditions to determine the best mechanistic pathways. In our study, oxidation of different secondary alcohols was done by using Benzimidazolium Fluorochromate in Dimethyl Sulphoxide, which is a non-aqueous solvent. Oxidation resulted in the formation of ketonic compounds. The reaction showed first order kinetics both in BIFC and in the alcohols. Hydrogen ions were used to catalyze the reaction. We selected four different temperatures to carry out our study. The correlation within the activation parameters like enthalpies and entropies was in accordance with the Exnerʼs criterion. The deuterated benzhydrol (PhCDOHPh) oxidation exhibited an important primary kinetic isotopic effect (kH / kD = 5.76) at 298 K. The solvent effect was studied using the multiparametric equations of Taft and Swain. There was no effect of addition of acrylonitrile on the oxidation rate. The mechanism involved sigmatropic rearrangement with the transfer of hydrogen ion taking place from alcohol to the oxidant via a cyclic chromate ester formation.
KEYWORDS:Alcohols; Benzimidazolium Fluorochromate; Chemical Kinetics; Correlation Analysis; Kinetic Isotope Effects; Oxidation; Reaction Mechanism
Download this article as:| Copy the following to cite this article: Arora B, Ojha J, Mishra P. Kinetics and Mechanism of Oxidation of Aliphatic Secondary Alcohols by Benzimidazolium Fluorochromate in Dimethyl Sulphoxide Solvent. Orient J Chem 2021;37(3). |
| Copy the following to cite this URL: Arora B, Ojha J, Mishra P. Kinetics and Mechanism of Oxidation of Aliphatic Secondary Alcohols by Benzimidazolium Fluorochromate in Dimethyl Sulphoxide Solvent. Orient J Chem 2021;37(3). Available from: https://bit.ly/3de6TKo |
Introduction
Organic reaction mechanism not only deals with the relationship between the molecular structure and chemical properties but tells us about the rate constants. Thus, the organic reaction mechanism is a multidisciplinary one which involves organic chemistry, physical chemistry and if some metal compound is involved also inorganic chemistry. Correlation analysis of structure and reactivity is of great importance as nature of the substituent can cause field effect, steric effect or resonance, which directly affects reaction rates in the redox process.It also gives us information about the reaction mechanism, transition state, nature of the intermediates, oxidizing and reducing species formed during the course of a chemical reaction.
Chromium (V1) is a versatile oxidant which is usually found in the chromic acid, metal dichromates and metal halo chromates. It is normally soluble in water and insoluble in most of the organic solvents. Many chromium oxidants have been used in both aqueous and non-aqueous media for the study of oxidation of various organic compounds 1. Cr (VI) reagents are known to be multi functional and can oxidize almost all the organic functional groups. Many chromium complexes have been prepared and used in the synthetic organic chemistry 2,3. Oxidation of secondary alcohols to the carbonyl compounds 4-7 by the oxidants containing chromium (VI), has been widely studied 8-11.BIFC is also a very mild and selective oxidizing agent12. Most of the oxidation by BIFC has been carried out in an aqueous medium. In this paper we report the oxidation of different secondary alcohols by BIFC in non-aqueous medium.We feel our study on correlation analysis of different reactions will not only establish mechanism of the reaction but also determine kinetic parameters of the reaction, nature of reactive species and effect of solvent. The literature survey has also revealed that the oxidation of secondary alcohols by BIFC in the non-aqueous medium has not been done and presented so far thus we have used dimethyl sulphoxide as a solvent for our study. The main purpose of our research was to study rate constants, the effects of solvent for oxidative surfaces and propose an appropriate mechanism for oxidation
Experimental
Materials
All the substrates (alcohols) taken were the commercial products of fluka.13 Anhydrousmagnesium sulfate was used to dry the liquid alcohols, which were then subjected to fractional distillation. Diphenyl methanol was purified by recrystallizingfrom ethyl alcohol. BIFC was preparedby the reported method8. Iodometric titration was usedto check its purity. Deuterated benzhydrol (PhCDOHPh) was prepared by the method available in the literature14. NMR spectra confirmed its purity of 96±5%. We have used P- toluene sulphonic acid(TsOH)asa hydrogen ions source. Purification of solvents was done by the methods available in the literature15.
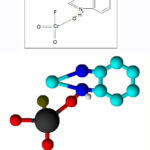 |
Scheme 1: Structure of Benzimidazolium Fluorochromate |
Product Analysis
We have taken0.1 mol secondary alcohols and 0.01 mol of BIFC and prepared its 100 mL solution using DMSO solvent. 0.5 mol dm-3of P-toluene sulphonic acid (PTS) was also added for the supply of H+to prevent photo-oxidation.Abovemixture was covered with a black paper and placed in the dark place for a day, till the reaction was complete. Then non-aqueous layer was separated, rinsed with H2O and was dehydrated using anhydrous magnesium sulphate8.The yields of the products were approximately 87%. The chromium oxidation state16 wasfound to be 3.92±0.14, in entirely reduced reaction mixture, which was ascertained by the iodometric titration method.
Kinetic measurement
For our experiments we used pseudo-first-order conditions, for which we took excess of alcohol over BIFC. DMSO was used as solvent, if other than specified.
All the studies were done at a constant temperature16,17 (± 0.1 K), and up to the 80% of reaction was observed by observing the decrease in the concentration of BIFC spectrophotochemically at a wavelength of 364 nm. Linear least squares method was used for the calculations of pseudo-first-order rate constant and a graph between log [BIFC] versus time was plotted. The reactions were repeated and it was observed that the rate constants were consistent with in ±3% limit18.
Results
Oxidation of secondary alcoholsby BIFC resulted in the production of ketones19. In general, overall reaction can be expressed as shown in the eqn. 1 (where BI = benzimidazolium).BIFC undergoes a 2-electron transformation which is clear from the below equation-
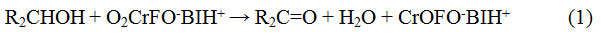
Rate law
The reaction order was found to be first with respect toBIFC.20,21 Figure (1) illustrates a typical kinetic run & Figure (2) represents order of reaction. Moreover, the rate constant was independent of the initial concentration of BIFC.The reaction rate increased directly with the increasing concentration of the alcohols22 (Table 1). The plot of 1/kobs versus 1/[alcohol] plot was linear with the slop on the rate ordinate23 suggesting the Michaelis – Menten type of kinetics with respect to the alcohols (Figure 3). This is represented by the Eqn. (2). Equation (3) is referred to the mechanism and Eqn. (4) assigns the rate law.
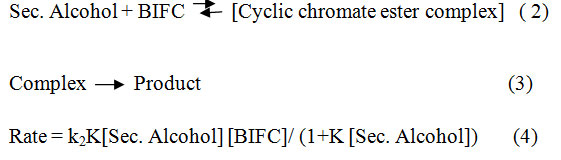
Induced polymerization testof Acrylonitrile
In the nitrogen atmosphere, the alcohols oxidation was ineffective to induce the acrylonitrile polymerization19. Moreover, there was no effect of addition of acrylonitrileon the oxidation rate. This clearly indicates that no free radical formation is observed during reaction. (Table1).
Table1: Rate constants obtained for the secondary alcohols oxidation by BIFC at 298 K
|
[Sec.alcohols] (mol L-1) |
103[BIFC] (mol L-1) |
[H+] (mol L-1) |
2-hydroxyPropane 104kobs/S |
2-hydroxyButane 104kobs/S |
2-hydroxyPentane104kobs/S |
2-hydroxyHexane 104kobs/S |
|
0.10 |
1.0 |
0.1 |
6.41 |
10.9 |
14.8 |
16.4 |
|
0.20 |
1.0 |
0.1 |
11.2 |
19.2 |
32.2 |
36.3 |
|
0.40 |
1.0 |
0.1 |
22.1 |
37.5 |
58.6 |
69.1 |
|
0.60 |
1.0 |
0.1 |
33.6 |
56.8 |
87.9 |
97 |
|
0.80 |
1.0 |
0.1 |
44.5 |
75.2 |
116 |
131 |
|
1.00 |
1.0 |
0.1 |
54.8 |
94.8 |
143 |
162 |
|
0.20 |
2.0 |
0.2 |
12.4 |
20.3 |
33.3 |
37.2 |
|
0.20 |
4.0 |
0.4 |
10.6 |
18.7 |
31.6 |
35.4 |
|
0.20 |
6.0 |
0.7 |
11.8 |
19.8 |
32.7 |
36.8 |
|
0.20 |
8.0 |
1.0 |
10.8 |
18.9 |
31.8 |
35.6 |
|
0.40* |
1.0 |
0.1 |
23.9* |
38.9* |
59.6* |
70.4* |
* contained 0.001 mol dm-3acrylonotrile.
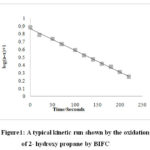 |
Figure 1: A typical kinetic run shown by the oxidation of 2- hydroxy propane by BIFC |
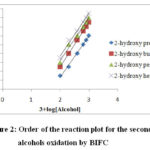 |
Figure 2: Order of the reaction plot for the secondary alcohols oxidation by BIFC |
 |
Figure 3: Secondary Alcohols oxidation by BIFC: |
Effect of Hydrogen ions
PTS was used for the supply of H+needed to increase the rate of the reaction. The rate of secondary alcohols oxidation was increased with the increase in the (H+) acidity as shown in the (Table 2)and Figure (4). The dependence of hydrogen ions can be represented by the following equation
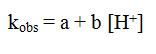
Table 2: Dependence of rate on [H+]
|
[Sec.Alcohol] 0.10 L-1 [BIFC] 0.001 mol L-1 Temperature 298 K |
||||||
|
[H+]/mol L-1 |
0.1 |
0.2 |
0.4 |
0.6 |
0.8 |
1.0 |
|
2-hydroxy propane/(104kobs/s-1) |
6.41 |
9.34 |
11.5 |
13.3 |
15.9 |
18.6 |
|
2-hydroxy butane/(104kobs/s-1) |
10.9 |
15.2 |
19.6 |
22.4 |
25.7 |
29.8 |
|
2-hydroxy pentane/(104kobs/s-1) |
14.8 |
19.2 |
25.5 |
31.7 |
35.9 |
41.6 |
|
2-hydroxy hexane/(104kobs/s-1) |
16.4 |
22.8 |
30.1 |
36.6 |
43.7 |
49.9 |
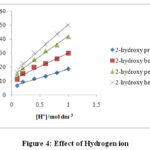 |
Figure 4: Effect of Hydrogen ion. |
Kinetic isotope effect
Primary kinetic isotopic effect24,25 was exhibited by the deuterated benzhydrol (PhCDOHPh) and kH/kDvalue of 5.76 was obtained at a temperature of 298 K20,21.A significant initial kinetic isotopic effect was demonstrated by the PhCDOHPh oxidation. This confirmed the fact that the cleavage of α-C-H bonds is taking place in the rate-determinationstep26. A significant initial kinetic isotopic effect was demonstrated by the PhCDOHPh oxidation (Table 3).
Influence of temperature on the reaction rate
The kinetic study of ten secondary alcohols oxidation has been done at different temperatures viz. 318,308,298 and 288K in DMSO solvent and the calculations of activation parameters has been done (Table 3). The log k2 at different temperatures was linearly related20,21 to 1/T in all the alcohols as shown in the (Figure 5), which verifies the Arrhenius equation27 for these oxidations.
Table 3: Enthalpy, entropy, free energy and rate constants obtained for the secondary alcohols oxidation by BIFC.
|
Rate constants at K Activation parameters |
|||||||
|
Sec. alcohol |
104k2/mol-1dm3s-1 |
∆H* |
∆S* |
∆G* |
|||
|
(R) |
288 |
298 |
308 |
318 |
(kJ mol-1) |
(J mol-1 K-1) |
(kJ mol-1) |
|
Methanol |
15.9 |
33.6 |
75.4 |
152 |
55.2±0.7 |
-31±2 |
64.2±0.6 |
|
Ethanol |
26.5 |
54.8 |
115 |
221 |
51.6±0.4 |
-39±1 |
63.1±0.3 |
|
Propanol |
46.0 |
87.9 |
167 |
289 |
44.4±0.4 |
-59±1 |
61.9±0.3 |
|
i-Propanol |
75.0 |
142 |
295 |
480 |
45.5±1.3 |
-51±4 |
60.7±1.0 |
|
i-Butanol |
124 |
223 |
412 |
673 |
40.8±0.6 |
-64±2 |
59.6±0.5 |
|
Butanol |
53.2 |
97 |
205 |
385 |
48.3±1.3 |
-45±4 |
61.5±1.0 |
|
ClCH2 |
0.24 |
0.69 |
1.64 |
4.20 |
69.4±0.9 |
-16±3 |
74.0±0.7 |
|
MeOCH2 |
2.02 |
4.85 |
11.2 |
25.4 |
61.6±0.4 |
-25±1 |
69.1±0.3 |
|
Phenol |
106 |
223 |
421 |
780 |
47.9±0.4 |
-40±1 |
59.7±0.3 |
|
Benzhydrol |
124 |
230 |
466 |
843 |
46.6±0.9 |
-44±3 |
59.4±0.7 |
|
Benzhydrol-a-d |
20.7 |
39.9 |
82.1 |
159 |
49.5±0.9 |
-48±3 |
63.8±0.7 |
|
kH/kD |
5.99 |
5.76 |
5.68 |
5.30 |
|||
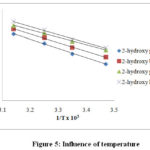 |
Figure 5: Influence of temperature. |
Influence of solvents
Oxidation of different secondary alcohols was studied in different organic solvents28. We have used many organic solvents as listed in the table (4). Due to the solubility 20,21 of BIFC and its reaction with alcohols the solvents choice was restricted 19. There was no reaction found with the selected solvent. The kinetics19 of all solvents was similar. Therefore, we have taken 2-hydroxy propane as the representative secondary alcohol. The value k2at 308 K is listed in Table 4. (cf. Fig.6)
 |
Figure 6: Secondary alcohols oxidation by BIFC: |
Table 4: Effect of Solvents on 2-hydroxy propane oxidation by BIFC at 308 K
|
Solvent |
104k2(s-1) |
Solvent |
104k2(s-1) |
|
Chloroform |
40.5 |
Ehanoic acid |
8.24 |
|
1,2-Dichloromethane |
45.3 |
Hexanaphthene |
1.46 |
|
1,1-Dichloromethane |
35.9 |
Methylbenzene |
10.9 |
|
Dimethyl sulphoxide |
75.4 |
Phenylethanone |
47.8 |
|
Acetone |
32.1 |
Tetrahydrofuran |
16.7 |
|
DMF |
66.1 |
Tert-butyl alcohol |
12.4 |
|
Butanone |
27.4 |
1,4-Dioxane |
13.2 |
|
Nitrobenzene |
50.1 |
1,2-Dimethoxyethane |
11.8 |
|
Benzene |
11.8 |
Carbon disulfide |
6.26 |
|
Ethyl acetate |
12.2 |
Discussion
The correlation29 within the activation parameters like enthalpies and entropies29 of the ten secondary alcohols oxidation was not satisfactory (r2 = 0.9391), indicating that the compensation effect was not operative in this reaction30. The correlativity was in accordance with the Exnerʼs criterion31-32. An Exnerʼs graph32 was plotted between log k2 at two different temperatures 298 K and at 318 K. The graph was linear and (r2) value was 0.9989 (Figure7). The isokinetic temperature 30 has the value 717±84 K, which was evaluated by using the Exnerʼs plot. The isokinetic relationship30 was determined and it suggested that all the secondary alcohols are oxidized by the same mechanism20,21 and the change in the reaction rate was controlled by both the changes in the enthalpy and entropy of the activation30.
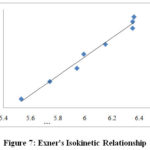 |
Figure 7: Exner’s Isokinetic Relationship. |
The oxidation rate constants, k2 in all the solvents used were correlated in the term oflinear solvation energy relationship (LSER) of Kamlet33 and Taft34.

In this equation, π* representedthe solvent polarity20,21, β the hydrogen bond acceptor basicityand α is the hydrogen bond donor acidity20,21. A0 is the intercept as per the standard line equation. It can be stated here that 12 out of 18 solvents had zero value for α. The correlation analysis results with respect to Eqn. (5), a parametric equation involving π* and β, and separately with π* and β are given below as equations (6) – (9).

Here nis the number of data points and ψ is the Exnerʼs statistical parameter32
However, the correlation was not satisfactory by Exner’s criterion32 (cf. eqn. 6). The main contribution was due to polarity of thesolvent29. It singly accounted for ca. 85% of the data29.Both β and α played relatively minor roles29. Solvent effect data were analyzed in relation to Swain’s equation35 (10), which included the cation and anion solvating36,37 conception of the solvent.

Where A denotes the anion-solvating power of the solvent and B denotes the cation-solvating power, C denotes the intercept term and (A+B) indicates the polarity of solvent33. The rate was determined in different solvents in terms of eqn. (10), by using A and B and separately with (A + B)36,37.
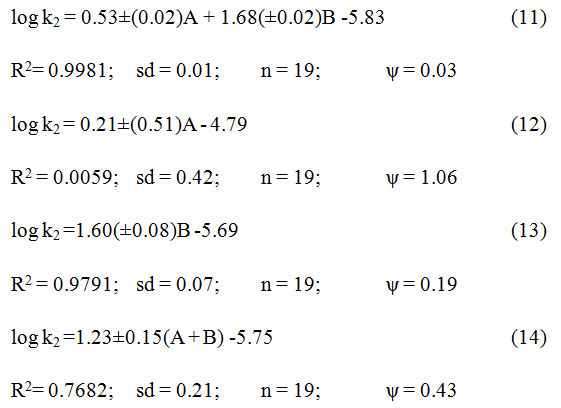
The rate of oxidation of 2-propanol and other secondary alcohols in different solvents16 showed excellent correlativity in terms of Swain’s equation35 (cf. eqn. 11), which plays a major role with cation solvating strength. In fact, cation solvating power accounts for 99% of the data. The correlativity with the anion solvating power was minimal. The solvent polarity indicated by (A + B), was also considered for 89% of the data.
Correlation analysis of reactivity
The rate of alcohols oxidation failed to give any important correlation separately with Taftʼs34 σ* and Es values.There was no significant co-linearity (r2 = 0.2332) between σ* and Es of the 8 substituents.
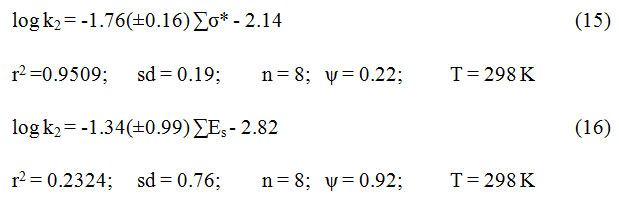
Therefore, the rates were correlated in terms of Pavelich-Taftʼs dual substituent-parameter equation38 (17).
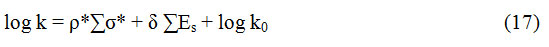
The correlativity was excellent as the reaction constants were negative as shown in the (Table 4). This supports the fact that in the intermediate state39 of the rate determination step, there is a carbon center with the electron deficiency. This negative steric hindrance indicated a steric acceleration of the reaction15. It can be described as based on the high ground state energy20,21 of highly crowded alcohol. The rapid oxidation of 1-phenyl ethanol and benzhydrol happened due to the stabilization of the carbon centre with electron-deficiency in the transition state by the phenyl group by resonance19.
Table 4: Correlation of rates of Oxidation in terms of Taftʼs equation
|
Temperature / K |
ρ* |
δ |
r2 |
sd |
ψ |
|
298 |
-1.75±0.02 |
-0.69±0.02 |
0.9998 |
0.02 |
0.015 |
|
308 |
-1.63±0.01 |
-0.60±0.01 |
0.9999 |
0.01 |
0.012 |
|
318 |
-1.56±0.01 |
-0.52±0.01 |
0.9998 |
0.01 |
0.014 |
|
328 |
-1.47±0.01 |
-0.44±0.01 |
0.9999 |
0.01 |
0.014 |
Mechanism
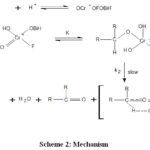 |
Scheme 2: Mechanism. |
In the present study due to the presence of considerable primary kinetic isotope effect14 (kH / kD = 5.76) at 298 K, it was confirmed that the cleavage of α-C-H bond was taking place in the rate-determining step24,25. The negative value of reaction constant and the considerable deuterium isotope effect both are indicative of the presence of an electron-deficient carbon centre in the transition state40. Therefore, the transfer of hydride-ion from secondary alcohol to the BIFC is suggested. The bigger role of cation-solvating power of the solvents also supports the hydride ion transfer mechanism41. The hydride ion transfer can occur either via an acyclic bimolecular reaction or may occur due to the involvement of a cyclic, planner and symmetrical transition state of chromate ester. The reaction is a two-step process, which was duly supported by the kinetic studies. Therefore, the whole mechanism is suggested to involve formation of the cyclic chromate ester15 formation in the rapid first phase. The slower second phase involves decomposition of the cyclic ester21,22 unit via a cyclic concerted symmetrical transition state via sigmatropic rearrangement leading to the formation of the product as represented above. The polar transition state is also supported by the observed negative value of entropy of activation28. The solvent effect has also indicated that the intermediate state is more polarized than the reactant40-48.Finally, we can say that BIFC is a very effective, mild oxidant, which can be used for the synthesis of ketonic compounds.
Acknowledgement
We are thankful to our HOD, Professor Kailash Daga, for providing laboratory facilities and chemicals needed for the conduct of this work, executed at the department of Chemistry, Jai Narain Vyas University, Jodhpur, India.
References
- Asghar, B. H.; Mansoor, S. S.; Hussain, A. M.; Malik, V. S.; Aswin, K.; Sudhan, S. P. N., Oxidation of aliphatic aldehydes by benzimidazolium fluorochromate in non aqueous medium- A kinetic and mechanistic study,Arabian J. Chem., 2017, 10(2), S-2115 – S-2123.
CrossRef - Mansoor, S. S.; Shafi, S. S., Oxidation of anilines and some para-substituted anilines by benzimidazolium fluorochromate in aqueous acetic acid medium- A kinetic and mechanistic study, Arabian J. Chem.,2014,7.171-176
CrossRef - Choudhary, K.; Agarwal,S.; Banerjee, K. K., Kinetic study of the oxidation of thioacids by pyridinium fluorochromate, Transition metal chemistry,1991,16(6),641-644.
CrossRef - Bhandari, A.; Mishra, P.; Banerjee, K. K., Kinetics and mechanism of the oxidative regeneration of carbonyl compounds from oximes by pyridinium fluorochromate,Reaction Kinetics Catalytic Letter, 2000, 71(2), 343-347.
CrossRef - Kumar, A.; Mishra, P.; Kotai, L.; Banerjee, K. K., Kinetics and mechanism of the oxidative regeneration of carbonyl compounds from oximes by tetraamminecopper (lll) permanganate, IJC, 2003, 42A, 72-74,
- Pohani, P.; Mishra, P.; Sharma, P. K., Kinetics and mechanism of the oxidation of some thioacids by benzyltriethylammonium chlorochromate, Int. J. Chem Sci,2005, 3(3), 461-468.
- Chouhan, K.; Mishra, P.; Sharma, P. K., Kinetics and mechanism of the oxidation of some unsaturated acids by benzyltriethylammonium chlorochromate,Int. J. Chem Sci, 2005, 3(2), 271-279
- Hudlicky, M.: Oxidations in organic chemistry” American Chemical society, 186, 2nd Ed. 1990.1,2,186.
- Fieser, L.; Fieser, M. Reagents for organic synthesis, John Wiley and Sons, INC., New York-London-Sydney, 1967, 1.
- Cainelli, G.; Cardillo, G. Chromium oxidation in organic chemistry, Springer-Verlag, New York,1984,19,1-7.
CrossRef - Vellaisamy, M.; Suryakala, K.; Ravishankar, M.Kinetics and mechanism of the oxidation of 2-naphthol by nicotiniumdichromate,J. Chem. Pharm. Res., 2011, 3(5), 678-681.
- Ozgun, B.; Degirmenbasi, N., Oxidation of substituted benzyl alcohols by quinoxalinium dichromate: A kinetic study,Monatshefte fur Chemie, 2004,135(5), 483-491.
CrossRef - Srinivasan, N. S.; Venkatasubramania N., Oxidation of alcohols by N-chlorosuccinimide- A kinetic study, Tetrahedron, 1974,30(3), 419-425.
CrossRef - Swami, P.; Malani, N.; Agarwal, S.; Sharma, P. K., Oxidation of aliphatic aldehydes by tetraethylammonium chlorochromate: A kinetic and mechanistic study, Prog. React. Kinet. Mec. 2010, 35(3), 309-319.
CrossRef - Perrin, D. D.; Armarego, L.; Perrin, D. R. Purification of organic compounds, (Pergamon, Oxford) 1966.
- Bishnoi, G.; Sindal, R. S.; Mishra, P.; Sharma, P. K., Kinetics and mechanism of the oxidative regeneration of carbonyl compounds from oximes by quinolinium bromochromate,J. Ind. Chem. Soc. 2007, 84(5), 458-461.
- Sharma, A.; Vyas, N.; Choudhary, A.; Prasadrao PTSRK; Sharma, V., Oxidative regeneration of carbonyl compounds from oximes by pyridinium fluorochromate: A kinetic and mechanistic study,Asian J. Chem., 2013, 25(5), 2792-2796.
CrossRef - Vyas, S.; Sharma, P. K.; Banerji, K. K., Kinetics and mechanism of the oxidation of some unsaturated acids by 2,2’-bipyridinium chlorochromate, Ind. J. Chem., 2001, 40A(11), 1182-1186.
- Sharma, P. K., Kinetics and mechanism of the oxidation of aliphatic secondary alcohols by benzyltrimethylammonium chlorobromate,Asian J. Chem. 2014, 26(9), 2702-2706.
CrossRef - Daiya, A.; Sharma, D.; Baghmar, M.; Mishra, P.; Sharma, S.; Sharma, V., Oxidation of aliphatic primary alcohols by tetrakis (pyridine) silver dichromate- A kinetic and mechanistic approach, Eur. Chem. Bull.,2012, 1(3-4), 75-80.
- Purohit, V.; Mishra, P., Kinetics and mechanism of oxidation of secondary alcohols by morpholinium fluorochromate,Eur. Chem. Bull., 2016, 5(8), 324-329.
- Baghmar, M., Kinetics and mechanism of oxidation of aliphatic aldehydes by tetrabutylammonium tribromide, Int. J. Chem. Kinet., 2001, 33(6), 390-395.
CrossRef - Kumar, R.; Pandey, D.; Kothari, S., Kinetics and mechanism of the oxidation of oxyacids of phosphorus by benzimidazolium dichromate, Prog. React. Kinet. Mec., 2011, 36(4), 352-360.
CrossRef - Westaway, K. C., Using kinetic isotope effects to determine the structure of the transition states of SN2 reactions,Adv. Phy. Org. Chem. 2006, 41, 217-273.
CrossRef - Simmons, E. M.; Hartwig, J. F., On the interpretation of deuterium kinetic isotope effects in C-H bond functionalizations by transition metal complexes,Ange. Chem. Int. Ed. 2012, 51(13), 3066-3072.
CrossRef - Knipe, A. C., Organic Reaction Mechanism, 2016″, Wiley, 2020.
CrossRef - Arhenius, S. A., On the reaction rate of the inversion of non-refined sugar upon souring, Z. Phys. Chem. 1889, 4, 96-116.
- Reichardt, C.; Welton, T., Solvents and solvent effects in organic chemistrty, Wiley-VCH, 1990, 147-181.
- Vadera, K.; Yajurvedi, D.; Purohit, P,; Mishra, P.; Sharma, P. K., Structure reactivity correlation in the oxidation of substituted benzaldehydes by pyridinium bromochromate,Prog. React. Kinet. Mec., 2010, 35, 265-280.
CrossRef - Liu. L.; Guo, Q. X., Isokinetic relationship, isoequilibrium relationship and enthalpy-entropy compensation, Chem. Rev. 2001, 101(3), 673-695.
CrossRef - Saraswat, S.; Sharma, V.; Banerjee, K. K., Kinetics and mechanism of oxidation of aliphatic primary alcohols by quinolinium bromochromate, J. Chem. Sci. 2003, 115(1), 75-82.
CrossRef - Exner, O., Additive physical properties. I. General relationships and problems of statistical nature, Collect.Czech. Chem. Commun., 1966,31(8),3222-3251.
CrossRef - Kamlet, M. J.; Doherty, R. M.; Abraham, M. H.; Marcus, Y.; Taft, R. W., Linear salvation energy relationship, J. Phys. Chem., 1988, 92, 18, 5244-5255.
CrossRef - Taft, R. W.; Abboud, J. L. M.; Kamlet, M. J.; Abraham, M., Linear salvation energy relations, Journal of solution chemistry, 1985.,14,153-186
CrossRef - Swain, C. G.; Swain, M. S.; Povel, A. L.; Alunni S., Solvent effects on chemical reactivity. Evaluation of anion and cation salvation components, J. Am. Chem. Soc., 1983,105(3),502-513.
CrossRef - Agarwal, A.; Bhatt, P.; Banerjee, K. K., Kinetics and mechanism of oxidation of organic sulphides by N-chloro acetamide,J. Phy. Org. Chem. 1989, 3, 174-180.
CrossRef - Vyas, S.; Sharma, P. K., Kinetics and mechanism of oxidation of organic sulphides by 2,2’-bipyridinium chlorochromate, J. Chem. Sci., 2002, 114(2), 137-148.
CrossRef - Pevelich, W. A.; Taft, R. W.,The evaluation of inductive and steric effects on reactivity. The methoxide ioncatalyzed rates of methanolysis of l-menthyl esters in methanol,Journal of American Chemical Society, 1957; 79(18):4935-4940.
CrossRef - Muller, P. Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994)”, Pure and Applied Chemistry, 1994.
CrossRef - Baghmaar, M., Kinetics and mechanism of oxidation of aliphatic alcohols by tetrabutylammonium tribromide, J. Chem. Sci., 2001, 113(2), 139-146.
CrossRef - Pandey, D.; Kachawa, T.; Kothari, S., Kinetics and correlation analysis of reactivity in the oxidation of some α-hydroxy acids by benzimidazolium dichromate, Prog. Reac. Kinet. Mec. 2018, 43(3-4), 300-314.
CrossRef - Choudhary K.; Sharma, P. K.; Banerji, K. K., Kinetics and mechanism of oxidation of aliphatic alcohols by quinolinium fluorochromate, Int. J. Chem. Kinet., 1999, 31(7), 469-475.
CrossRef - Purohit, V.; Mishra, P., Kinetics and mechanism of different α-hydroxy acids oxidation by morpholinium fluorochromate,Inn. Sci. and Soc. Tech., Cibtech, 2018, 6(1), 27-37.

This work is licensed under a Creative Commons Attribution 4.0 International License.











0 Comments