Cement Chemisrty: Hydration of Ca2-Xsrxsio4 Compound
Antikolonial Prodjosantoso1, Wahyu Widiyati1, Wafin1, Ani Widyawati2 and Maximus Pranjoto Utomo1
1Department of Chemistry, Yogyakarta State University, Yogyakarta, DIY 55281, Indonesia.
2Departmentof Science Education, UniversitasSarjanawiyataTamansiswa, Yogyakarta, DIY 55165, Indonesia.
Corresponding Author E-mail: prodjosantoso@uny.ac.id
DOI : http://dx.doi.org/10.13005/ojc/370310
Article Received on : 03-Apr-2021
Article Accepted on :
Article Published : 31 May 2021
Dicalcium silicate (Ca2SiO4) isan importantcomponent of cement. The compound ofCa2-xSrxSiO4 can be formed if the Sr containing precursorsare used to synthesize the cement. The presence of Sr may alter the hydrationof the product. The hydration chemistry ofCa2-xSrxSiO4 compounds is reported.
The hydration of Ca2-xSrxSiO4was conducted uder nitrogen atmosphere for about 6 months. The dry samples were characterized usingXRD, FTIR, TGA-DSC, andSEM-EDXmethods.
It is confirmed that the hydration ofCa2-xSrxSiO4produces mainlyCa3Si2O7.3H2O and Ca(OH)2. However, the Sr doped Ca3Si2O7.3H2O andCa(OH)2 are possibly formed.The compound of CaCO3,as the result ofinteractions between Ca(OH)2 and atmospheric CO2gas during the sample handling,is also observed.
Cement; Ca2-xSrxSiO4; Ca(OH)2; Dicalcium silicate; Hydration
Download this article as:| Copy the following to cite this article: Prodjosantoso A, Widyawati W, Wafin w, Widiyati A, Utomo M. P. Cement Chemisrty: Hydration of Ca2-Xsrxsio4 Compound. Orient J Chem 2021;37(3). |
| Copy the following to cite this URL: Prodjosantoso A, Widyawati W, Wafin w, Widiyati A, Utomo M. P. Cement Chemisrty: Hydration of Ca2-Xsrxsio4 Compound. Orient J Chem 2021;37(3). Available from: https://bit.ly/3i1V1P7 |
Introduction
Cement is a hydraulic compound which able to bind other solid materials, forming a hard and insoluble solid mass unit. In practice, the cement is applied to agregate stones, bricks, concrete blocks, ceramics or more other building materials. Active compounds in Portland cement include calcium silicate. If lime and silica are mixed thoroughly and heated, four different calcium silicate compounds will be formed, one of which is dicalcium silicate (Ca2SiO4), which has five forms of polymorphism, namely α, α’H, α’L, β and γ.1
Portland cement is made of a mixture of minerals containing various elements including Mg, Ca, Si, and Al. However,a trace of Sr may be present in the minerals. So there is a possibility that the element of Sr is doped in the Ca2SiO4producing Ca2-xSrxSiO4 compound. This is supported by Bickle (1994) stated that the compound of Sr doped in β- or α’L-Ca2SiO4 can be formed in the cement if Sr is present in the cement raw materials.2
In use, the cement is usually mixed with water, so that it forms hardened cement hydrate. The hydration of dicalcium silicate can only be studied if the hydrate form is stable as a solid solution. The hydration rate of dicalcium silicate is varied. Very slow rate of hydration of γ-Ca2SiO4 relative to other Ca2SiO4 polymorphs is caused by the position and arrangement of oxygen atoms around Ca2+ ions in γ-Ca2SiO4 which is irregular compared to other polymorphs.3This indicates that the Ca2SiO4 hydrolysis depends on the thermodynamic factors, especially the lattice energy and hydration heat. The hydration products of β-Ca2SiO4 are Ca3Si2O7.3H2O and portlandite (Ca(OH)2).4,5In the air, Ca(OH)2may interact with CO2and H2O. It is well known that the compound of calcium hydroxide is not very stable in the concrete, and will usually react with other components to form a more stable structure.6–8
Elements that are doped in the compounds mayalter the nature and the activity of other compounds, such as calcium silicate hydration. The hydration reaction of Ca2-xSrxSiO4 compounds has not been studied. The hydration of Ca2-xSrxSiO4 under a nitrogen gas environment will be studied.
Experimental
This research was conducted to examine the hydration reaction of Ca2-xSrxSiO4(x = 0, 0.01, 0.025 and 0.05)compound. The hydration process was carried out by adding distilled water to the Ca2-xSrxSiO4compound,with weight ratio of 100:1, in the sample bottle. The mixture was stirred and bubled with nitrogen gas to remove CO2from the mixture. After the free CO2 mixture was obtained, the sample bottle was immediately tightly closed so that the mixture ofCa2-xSrxSiO4was isolated from the air. Then the mixture was allowed to stand for 6 months, and followedby drying at 110 °C to evaporatethe water to obtain dryCa2-xSrxSiO4hydrated. The characterization of the hydrated Ca2-xSrxSiO4 was undertaken by using powder X-Ray Diffraction (XRDBruker D2 Phaser), SEM-EDX (JEOL IT300), FTIR (Thermo Nicolet IS 10) and TGA-DSC (Linseis) methods.
Result and Discussions
The X-Ray Diffraction Spectroscopy method has beenapplied to determine the qualitative aspects of components in the hydrated Ca2-xSrxSiO4(x = 0, 0.01, 0.025 and 0.05)compounds. The X-ray diffraction patterns of the samples are depicted in Figures 1 and 2.
The XRD diffraction patterns of hydrated samples (Figures 1 and 2) indicate that the hydration reaction is expected to occur in Ca2-xSrxSiO4. The hydration reactionshappensimilar to the hydration of Ca2SiO4equations as follows.
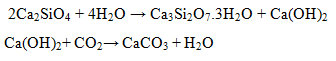
The hydration of Ca2-xSrxSiO4compound produces Ca3Si2O7.3H2O and Ca(OH)2, and it is also believed that the isomorphous of Ca3Si2O7.3H2O that is Ca3-xSrxSi2O7.3H2O exists. The XRD spectra also indicates the presence of CaCO3 compound. This is possible because of the samples are not always isolated under the nitrogen gas. Calcium hydroxide is relatively unstable in the open air, and so it reacts easily with other compounds in the air, one of which is CO2 and forms calcium carbonate. In addition, unreacted Ca2-xSrxSiO4 compound is also observed. This is possible because the hydration of Ca2-xSrxSiO4compound is very slow and happened from the outer to inner layer, leaving the inner part of Ca2-xSrxSiO4unreacted.
 |
Figure 1: The X-ray diffraction patterns of hydrated Ca2-xSrxSiO4 compounds withx = 0 (a), 0.01 (b), 0.025 (c), dan 0.05 (d). |
 |
Figure 2: The X-ray diffraction patterns of |
The IR spectra of hydrated Ca2-xSrxSiO4compounds are depicted in Figures 3 and 4. The Si-O streching vibrations is observed in the low and narrow band located at ~ 800 cm-1. Thisis in agreement with the finding of Ping et al., stated that the vibration of Si-O stretchingappears in the region of 795-800 cm-1, and the Si-O stretching from SiO4 tetrahedron is located in the region of 800-1000 cm-1.9,10The band position may shift to higher or lower wave number according to the ratio of calcium/silica.
The Si-O-Si asymmetric stretching of Ca2-xSrxSiO4 compound with different variations of x are observed at peak of ~ 1000 cm-1 with height and width shaped bands. The intensity can be related to the progress of hydration.11,12
The O-H stretchings in free water appear with low and wide band at wave number ~ 3400 cm-1.13,14 Absorption at ~ 3640 cm-1represents O-H stretching of Ca/Sr(OH)2 compound.This weak and wide peakis overlaped with OH’s streching bandof free water. The weak and narrow band at ~ 874 cm-1represents Ca/Sr-O bond.15
The bands at 1400-1600 cm-1 indicate the presence of CaCO3.The formation of CaCO3 is a complex process involving reactions on theCa(OH)2 particle surfaces. The mechanism of this reaction is not yet fully understood. In the early stages the carbonate formed could be considered amorphous, which then is developed to crystalline phase. The C-O bending molecular vibrationisobserved as sharp and high band at ~ 1480 cm-1, while the band in the region of 1483 cm-1 belongs to the CO32- group.Vibration of the C-O bending is observed at ~ 850 cm-1, which is the region of the bending vibration of the carbonate group.16A very small band at 2927 cm-1 can be associated with calcite vibrations of C-O.17
 |
Figure 3: The IR spectra of hydrated Ca2-xSrxSiO4compounds with x = 0 (a), 0.01 (b), 0.025 (c), dan 0.05 (d). |
 |
Figure 4: The IR spectra of hydrated Ca1.975Sr0.025SiO4 compound. |
The thermogravimetric analysis (TGA) is undertaken onabout 22 mg ofhydrated Ca2-xSrxSiO4 sample. TGA measurement shows that the typical thermal decomposition of hydrated Ca2-xSrxSiO4 compound occurs in several steps as shown in Figure 5.
The thermal decomposition of theall hydratedCa2-xSrxSiO4 compounds occurs at similar temperature. The compounds are estimated to undergo water releasing in the Ca3-2xSr2xSi2O7.3H2O at 100-165 °C, to form Ca3-2xSr2xSi2O7 and free H2O. The reaction is as follows.
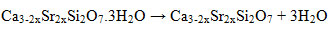
Decomposition continues to a temperature of ~ 600 °C in which the estimated loss of water in the Ca(OH)2producing CaO. In theory, the decomposition of Ca(OH)2 occurs at 240-550 °C. The decomposition of Ca(OH)2 compound is less in accordance with the theory, presumably because the water in Ca(OH)2 compounds is very crystalline so it requires longer time and higher temperature to release. The Ca(OH)2 decomposition can be described as follows.
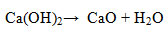
The presence of CaCO3 compounds in the sample can be made possible because Ca(OH)2 is a reactive compound and is not always in an open CO2-free condition. The decomposition of the carbonateoccursat a temperature of 750-780 °C which is the loss of CO2from the CaCO3 compound. The reactions are:
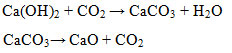
 |
Figure 5: The typical TGA (a) and DSC (b)curves of the hydrated Ca1.975Sr0.025SiO4 compound. |
The Scanning Electron Microscopy Electron-Dispersive X-Ray Analyzer (SEM-EDX) is used to determine the surface morphology and composition of the samples of hydratedCa2-xSrxSiO4 with x = 0, 0.01, 0.025 and 0.05.
The SEM (Figure 6) indicates that the hydrated Ca2-xSrxSiO4 compound havingan irregular shape, withthe size at about 0.3 µm to 0.67 µm in diameter, and a rough surface structure. The EDX spectra (Figure 7) shows the composition on the surface of hydrated Ca2-xSrxSiO4 compound under study. Selected EDX spectra of hydrated Ca2-xSrxSiO4 compound can be seen in Figure 7. Based on the EDX analysis, the quantity of Ca, Sr and Si atoms in the sample is obtained. However, the EDX analysis cannot be used as a basis for determining the number of atoms in the sample, because in general the EDX analysis is only done on the surface of the sample.
 |
Figure 6: The SEM images of thehydratedCa2-xSrxSiO4 compound with x = 0 (a), 0.01 (b), 0.025(c) and 0.05 (d). |
 |
Figure 7: EDX Spectraof hydrated Ca2-xSrxSiO4 with x = 0.025. |
Conclusions
The compound of Ca2-xSrxSiO4 has been hydrated producing at least two major products, that are Ca3-2xSr2xSi2O7.3H2O dan Ca(OH)2. TheCa(OH)2is partly carbonated to CaCO3. The products have been confirmed by existences of Si-O stretching, O-Si-O strecthing, O-H stretching, Ca-O stretching dan C-O bending dan stretching bands.The molecules of water of Ca3-2xSr2xSi2O7.3H2O are released during heating at 100-165°C, while heating at 600-650°C causes the decomposition of Ca(OH)2 producing CaO. The CO2 gas is produced by the heating CaCO3 at 750-780oC.
Acknowledgement
The authors would like to thank the Indonesian Ministry of Education and Culture for the funding of this research.
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this article.
References
- A.Prodjosantoso and cB. Kennedy, Turkish J. Chem.41, 4 (2017).
CrossRef - M. J.Bickle, Nature367, February (1994) doi:10.1038/367699a0.
CrossRef - F. Lea, The chemistry of cement and concrete. (2004).
- S. H.Hong andJ. F.Young, J. Am. Ceram. Soc.82, 7(1999) doi:10.1111/j.1151-2916.1999.tb01986.x.
CrossRef - K.Yanagisawa, X.Hu, A.Onda andK.Kajiyoshi, Cem. Concr. Res.36, 5 (2006).
CrossRef - G.Montes-Hernandez, F.Renard, N.Geoffroy, L.Charlet andJ.Pironon, J. Cryst. Growth308, 1 (2007) doi:10.1016/j.jcrysgro.2007.08.005.
CrossRef - C.Domingo, E. Loste, J.Gómez-Morales, J.García-Carmona andJ.Fraile, J. Supercrit. Fluids36, 3 (2006) doi:10.1016/j.supflu.2005.06.006.
CrossRef - P.H.R.Borges, J.O.Costa, N. B. Milestone, C.J.Lynsdale andR.E. Streatfield, Cem. Concr. Res.40, 2 (2010) doi:10.1016/ j.cemconres. 2009.10.020.
CrossRef - Y.Ping, R.J. Kirkpatrick, P. Brent, P.F. McMillanandX. Cong, J. Am. Ceram. Soc.82, 3 (1999) doi:10.1111/j.1151-2916.1999.tb01826.x.
CrossRef - M. Tomozawa, J.W. HongandS.R.Ryu, Journal of Non-Crystalline Solids351, 12-13 (2005). doi:10.1016/j.jnoncrysol.2005.01.017.
CrossRef - T.F.Sevelsted andJ. Skibsted, Cem. Concr. Res.71, May (2015) doi:10.1016/j.cemconres.2015.01.019.
CrossRef - F.Pelisser, P.J.P. Gleize andA. Mikowski, J. Phys. Chem. C116, 32 (2012) doi:10.1021/jp302240c.
CrossRef - T. Iwasita andX. Xia, J. Electroanal. Chem.55, 4 (1996) doi:10.1016/0022-0728(96)04576-7.
CrossRef - Ł.Szyc, J.R. Dwyer, E.T.J.Nibbering andT. Elsaesser, Chem. Phys.357, 1-3 (2009) doi:10.1016/j.chemphys.2008.08.013.
CrossRef - H.D.Lutz, H. Möller andM.Schmidt, J. Mol. Struct.322, (1994) doi:10.1016/0022-2860(94)08355-X.
CrossRef - A.K.Pathak andD.K.Maity, J. Phys. Chem. A454, 1-3 (2009) doi:10.1021/jp907577j.
CrossRef - A.M.Kalinkin, E.V.Kalinkina, O.A.Zalkind andT.I.Makarova, Inorg. Mater.41, (2005) doi:10.1007/s10789-005-0263-1.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










0 Comments