Using of Sukary and Khlass Date Pits as a Bio-adsorbents for Adsorption of Lead and Copper Ions from Waste Water
Department of Chemistry, College of Science, Al-Imam Muhammad ibn Saud Islamic University (IMSIU), P.O. Box 90952, Riyadh, Saudi Arabia.
Corresponding Author E-mail: agalhamzani@imamu.edu.sa
DOI : http://dx.doi.org/10.13005/ojc/370206
Article Received on : 01-Sep-2020
Article Accepted on :
Article Published : 10 Mar 2021
In this study date pits of two types of date-palm trees (Phoenix Dactylifera L.), in Saudi Arabia were used as bio-sorbents for heavy metals (e.g. lead and copper) from aqueous solutions. Investigation of equilibrium time and the effect of different concentrations of metals were performed. Adsorption capacity of bio-sorbents increased when increasing concentration of metal ions. Maximum adsorption capacity at room temperature of Sukary date pits was 17.53 mg g-1 and 9.86 mg g-1 for lead and copper ions, respectively. Whereas, Khlass date pits showed maximum adsorption capacity at 14.1 mg g-1 and 7.91 mg g-1 for lead and copper ions, respectively at room temperature. Equilibrium isotherm models, (Langmuir and Freundlich models), were used for analysis of equilibrium experimental results. these models describe the experimental data well.
KEYWORDS:Adsorption; Bio-Sorbents; Date Pits; Date-Palm Tree; Equilibrium Isotherm
Download this article as:| Copy the following to cite this article: Alhamzani A. G. Using of Sukary and Khlass Date Pits as a Bio-adsorbents for Adsorption of Lead and Copper Ions from Waste Water. Orient J Chem 2021;37(2). |
| Copy the following to cite this URL: Alhamzani A. G. Using of Sukary and Khlass Date Pits as a Bio-adsorbents for Adsorption of Lead and Copper Ions from Waste Water. Orient J Chem 2021;37(2). Available from: https://bit.ly/3l3gDKi |
Introduction
Lead and copper are heavy metals among the most harmful of the elemental pollutants because of their toxicities to humans. Main sources of these metals in wastewater are metal plating and industrial waste1 (He et al. 2006). At low levels might be essential, but at high levels they become toxic in particular in the drinking water and wastewater. They cause serious health problems to human being such as liver damaging and asthma-like allergy, thyroid, heart failure, and for aquatic life, their toxicity may be harmful to wildlife2 (WHO 1996).
Presently, many technologies such as membrane process3 (Rahmanian et al. 2011), chemical precipitation4,5 (Fu et al. 2011; Mauchauffee et al. 2007), ion exchange processes6, and adsorption7 are in use to remove heavy metals such as copper and lead from different sources of waters. But most of these techniques for the adsorption of heavy metals are not cost effective. Therefore, low-cost adsorbents are adequately for adsorption process to adsorb heavy metals like copper and lead ions from drinking and wastewater.
Recently, bio-sorbents with low costs attracted many researchers to develop them with high adsorption capacities to remove heavy metals8,9. Among these techniques, prepared activated carbon from pits of date-palm trees is considered cost effective for removing metal ions as compared to other costly methods such as ion exchange, electro-deposition, membrane filtration, electrochemical treatment, .. etc. Currently, there are lot of naturally occurring materials that may be used without treatment or by converting to activated carbon namely coconut shell is a common example10. In the Kingdom of Saudi Arabia there are more than twenty-eight million date palm trees, (Phoenix Dactylifera L.), which produce more than half a million tons of dates annually11. The primary waste of date fruit is the date pits.
Few studies have investigated the adsorption of heavy metals and dyes via using activated carbon produced from date pits 12-18. Attia12 showed that activated carbon produced from date pits has high surface area when carbonated at 500° C in a limited supply of air followed by activation with oxidizing gases such as steam. Girgis et al.13 reported that activated carbon from date pits prepared by chemical activation with phosphoric acid followed by pyrolysis at high temperature (700° C) has good adsorption capacity and best porosity.
A comparison study of investigation of efficiency of activated palm-tree date pits and non-activated one as bio-adsorbents for zinc and copper ions has been reported using batch adsorber.14 It was reported that the adsorption of ions was maximum on non-activated one. In another study, activated carbon that produced from date-palm tree pits was used for adsorption zinc.15 They observed high adsorption capacity at more than 9 milligrams per one gram. Further adsorption of phenolic compounds and methylene blue were investigated on activated carbon produced from pits of date-palm trees in Al-Ain city by Abdulkarim et al.16. They reported that the adsorption capacity of methylene blue was 590 mg g-1. Additionally, the activated carbon was suitable for the removal of phenolic compounds from solutions. Similarly, produced activated carbon and natural pits were used for adsorption of cadmium ions17. It was found that non-activated date pits showed ions sorption capacity greater than the activated one from solution, by two to three times. In Al-ain city, activated carbon has been prepared from palm-tree date pits and was used for adsorption lead ions and methylene blue from aqueous solutions.18 Other researchers reported that the adsorption capacity was very high into prepared activated carbon from date pits for methylene blue and lead ions, 220 mg g-1 and 23 mg g-1 respectively, at pH=5. Adsorption of lead (II), cadmium (II), Iron (III), and Strontium (II) ions via activated carbon produced from pits of date-palm trees was studied in Egypt in aqueous solution19 . In this investigation, they observed that some anions such as NO3-, CO3-2, SO4-2 in wastewater were affected the removal process of the metals. Evaluation of the chemical composition was carried on the extraction of oil fatty acids via date-pulp seeds in Tunisia20. The unsaturated fatty acid was linoleic acid which was 32.77% for the pulp and 47.66% for the pits. Types of saturated fatty acids were palmitic acid and the lauric acid, and the maximum adsorption was twenty percent for the pulp and seventeen percent for the pits.Modification of date pits using different types of acid such as tartaric acid, carboxylic acid, salicylic acid, mandelic acid, oxalic acid, and malic acid was done in Jordan for using them in the adsorption of copper, zinc, and cadmium from waste water 21. The study showed that adsorption of these metal ions was better via the modified than unmodified date-pits. Furthermore, the modified adsorbents namely SA-PS, CA-PS and MA-PS proved efficient adsorbents in removing these ions. For multi-components adsorbing system, the adsorbates OA-PS and TA-PS are the best adsorbents for removal of heavy metal ions.Date pits obtained from Eastern Province, Saudi Arabia were converted to activated carbon and utilized for the adsorption of methylene blue from wastewater. It was found that the activated carbon is very suitable for adsorption of methylene blue in a high adsorption capacity.22 In another study, activated carbon obtained from date-pits from Eastern Province, Saudi Arabia was used for the adsorption of three metals ions (e.g. lead, copper, and cadmium) from wastewater.23 This study showed that the capacity of adsorption was higher, for lead(II), than the copper and cadmium in descending order.
Some researchers used activated carbon prepared from date-pits from Eastern Province, Saudi Arabia and olive stones to adsorb methylene blue and compared it by photoactive catalysts24. It was found that adsorption of methylene blue was more efficient by photocatalytic method. However, the studies regarding the adsorption of heavy metals and dyes by Saudi date pits do not seem to be comprehensive13-15. Because, the effect of different types of date pits, their physio-chemical characteristics and performance for the removal of days and heavy metals is not investigated thoroughly. Besides, previous studies were mainly to convert date pits into activated carbon regardless of the type of the pits and no attempt was made to test the raw date pits for adsorption of heavy metals pollutants 13-15. It is well known that date pits have different chemical composition depending on the type of the date pits and its origin12,13,14. It is a hypothesis of this investigation that the amount of adsorption of metal ions will be affected by the type of the date pits. Since, in Saudi Arabia, date pits are easily and readily available.Therefore, study of non-biodegradable heavy metals adsorption with two famous types of natural Saudi date pits obtained from Sukary and Khlass date fruits as adsorbents may determine whether it is a desirable substitute for commercial activated carbon. Therefore, the aim of this research was to conduct a thorough investigation on two types of famous Saudi date pits namely Sukary and Khlass as adsorbents for the removal of heavy metal ions such as copper and lead from drinking and wastewater.
Materials and Methods
Materials
Two types of date-pits obtained from Sukary and Khlass of date-palm tree fruits were collected from Riyadh city, Saudi Arabia. Lead and copper solutions were prepared from lead (II) nitrate and copper (II) sulphate supplied by VWR chemicals.
Equilibrium Experiments
Date pits were grinded to a size of 0.125 mm. Then, lead solutions prepared with concentrations from 50 to 1400 ppm. For each 50 mL solutions of lead ions one gram of grinded date pits was added. The bottles were placed in a shaker. For isotherm run, the temperature was maintained at 20oC. The equilibrium experiments ran for 3 hrs to make sure that the process of adsorption reached astatic equilibrium. Then, the solid residue of grinded pits was removed by filtration using filter papers. The filtrate of each solution, which contains lead ions, was measured using AAS to indicate the concentrations of lead ions. The amount of lead adsorption, qe, was calculated using the following equation:
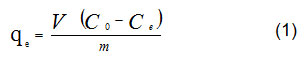
Where, (qe ) is the adsorption’s amount in mg g-1, (V) is the solution’s volume of in L, (Co ) is the initial concentration of lead in mg L-1, (Ce ) is the lead concentration at equilibrium in mg L-1, and (m)is the mass of grinded date pits in g. Isotherm curves of equilibrium adsorption were graphed by plotting amount of adsorbates (Lead and Copper ions) after adsorption against the initial ions’ concentration.
Results and Discussion
Equilibrium time
Figures 1 and 2 shows the equilibrium time for the adsorption. After 50 minutes, state of equilibrium was reached.
 |
Figure 1: Equilibrium time for copper adsorption on Sukary and Khlass date pits. |
 |
Figure 2: Equilibrium time for lead adsorption on Sukary and Khlass date pits. |
Initial concentrations Effect
Effect of initial concentrations on adsorption process was experimentally conducted. Figures 3 and 4 show the Equilibrium isotherm for adsorption ions. I noticed that the adsorption capacity of the tow ions increases as initial concentrations of ions increase. Maximum adsorption capacity at room temperature was found to be 17.53 mg. g-1 and 9.86 g.g-1 for lead and copper ions, respectively, when using date pits of Sukary as adsorbents. On the other hand, adsorption capacity was reached 14.1 mg.g-1 of lead and 7.91 mg.g-1 of copper when using pits from Khlass dates. Presence of carboxylic acid (-COOH) functional groups in the components of date-pits electrostatically attract the cation ions (lead and copper) in the solution.17
 |
Figure 3: Equilibrium isotherm of lead adsorption on Sukary and Khlass date pits. |
 |
Figure 4: Equilibrium isotherm of copper adsorption on Sukary and Khlass date pits. |
Analysis of Results from Equilibrium Experiments
Langmuir28 and Freundlich29 models, as equilibrium isotherm models, were used for analysis of equilibrium experimental results.
Langmuir isotherm
To estimate the maximum capacity of adsorption, Langmuir isotherm model, (equation 2) was applied. The model assumes that adsorbates form a monolayer on the adsorbents’ particles.

The linear form of Langmuir model is:

non-linear regression method (equation 3) was used to calculate equilibrium parameters (b and K).
The relationship between Ceand qeat temperature of 20 oC was tested, as shown in Figures 5 and 6. Then, Kand b values (Table 1) were calculated using equation 3. In Figure 5, experimental data fit well with Langmuir model.
 |
Figure 5: Langmuir model for lead ions adsorption on datepits of Sukary and Khlass. |
 |
Figure 6: Langmuir Model of adsorption copper ions on Sukary and Khlass date-pits. |
Table 1: Parameters of Langmuir Equilibrium Model for the adsorption of ions using date-pits.
|
a. Lead ions |
b. Copper ions |
||||||
|
Type |
K (lit/g) |
b (lit/mg) |
R2 |
Type |
K (lit/g) |
b (lit/mg) |
R2 |
|
Sukary |
0.2658 |
0.014725 |
0.977 |
Sukary |
0.3702 |
0.036502 |
0.988 |
|
Klass |
0.1361 |
0.00922 |
0.948 |
Klass |
0.0547 |
0.0054 |
0.923 |
Freundlich Isotherm
The Freundlich model, (equation 4), was used to explain results from determination of equilibrium adsorption for heterogeneous surface,
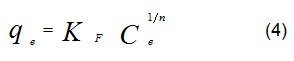
The linear form of the equation 4 is:
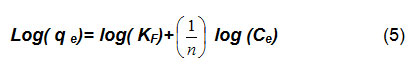
KF and parameters were calculated from equation (4) with nonlinear regression. As reported14, when n values are greater than one, that is mean cation ions favor to be adsorbed into adsorbents surface (i. e., date-pits).
Plotting LogCe verses Logqe(Figures 7 and 8), we can see that there is relationship between ions concentration (Ce) and the adsorption amount (qe) at temperature of 20 oC.The values of (KF and n ) are shown in Table 3, and they were calculated using nonlinear regression equation. Figures 7 and 8 show that the Freundlich isotherm well-correlates the data very well.
 |
Figure 7: Linear form of Freundlich isotherm model for the adsorption of lead ions. |
 |
Figure 8: Linear form Freundlich isotherm model for the adsorption of copper ions. |
Table 2: Equilibrium constants, KF and n, of Freundlich Isotherm Model for the adsorption lead and copper Ions.
|
Date Type |
Lead ions |
Copper ions |
||||
|
KF (Lit/g) |
n |
R2 |
KF (Lit/g) |
n |
R2 |
|
|
Sukary |
2.926 |
3.824092 |
0.932 |
5.9630 |
3.388 |
0.906 |
|
Khlass |
3.916 |
2.286 |
0.891 |
1.899 |
3.586 |
0.951 |
Conclusions
Pits of sukary and khlass dates are by-product of dates fruits in abundance in our local environment. Advantage has been taken from this that they are low-cost adsorbents.Effect of initial concentration of copper and lead ions as adsorbates into adsorbents (two Saudi date-pits namely Sukary and Khlass) was studied. The results of this work showed that the saturation capacity was in increased with the corresponding increase in the initial concentration of adsorbates (copper and lead ions) in solution.
A maximum adsorption capacity was observed when using date-pits of Sukary type at 9.86 mg g-1 and 17.53 mg g-1 for copper and lead ions, respectively at room temperature. Whereas, date-pits of Khlass type gave a maximum adsorption capacity at the concentration of 7.91 mg.g-1 and 14.1 mg.g-1 for copper and lead ions, respectively at room temperature. Application of Langmuir and Freundlich equilibrium isotherm models described the experimental data very well.
Aknowledgment
The author extend his appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number IFG-IMAMU-0701.
References
- He, Z.; Yang, X. E; Stoffella, P. J. J. Trace Elem. Med. Biol. 2005, 19(2–3), 125–140.
CrossRef - World Health Organization, Trace Elements in Human Nutrition and Health. Geneva, Switzerland (1996).
CrossRef - Rahmanian, B.; Pakizeh; M.; Esfandyari, M.; Heshmatnezhad, F.; Maskooki, A. J. Hazard. Mater. 2011,192, 585–592.
CrossRef - Fu, F.; Wang, Q. J. Environ. Manag.2011, 92, 407–418.
CrossRef - Mauchauffée, S.; Meux, E. Chemosphere, 2007, 69, 763–768.
CrossRef - Lai, Y. C.; Chang, Y. R.; Chen, M. L.; Lo, Y. K.; Lai, J.-Y.; and Lee, D. J. Bioresour. Technol.2016, 214, 192–198.
CrossRef - Davarnejad, R.; and Panahi, P. Sep. Purif. Technol.2016, 158, 286–292.
CrossRef - Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y. H.; Indraswati, N.; and Ismadji, S. J. Hazard. Mater.2009, 162, 616–645.
CrossRef - Ofomaja, A.E.; and Naidoo, E.B. Carbohydr. Polym. 2010, 82, 1031–1042.
CrossRef - Singha, B. and Das, S. K. Adsorption, 2012, 18, 395–401.
CrossRef - Awwad, N. S.; Daifuallah, A. A. M.; Ali, M. M. S.Solvent Extraction and Ion Exchange. 2008, 26, 6, 764-782.
CrossRef - T. M. Elmorsi, Journal of Environmental Protection,2011, 2,6, 817–827.
CrossRef - N. Ayawei, S. S. Angaye, D. Wankasi, and E. D. Dikio, Open Journal of Physical Chemistry, 2015, 5, 03, 56–70.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










