Universal pH Indicator as a Colorimetric Reagent for Differentiating Inorganic Anions
Department of Physical Sciences and Mathematics, College of Arts and Sciences, University of the Philippines, Manila, Philippines 1000
Corresponding Author E-mail: vgorgano@up.edu.ph
DOI : http://dx.doi.org/10.13005/ojc/360312
Article Received on : 16 May 2020
Article Accepted on : 23 Jun 2020
Article Published : 17 Jun 2020
A simple colorimetric approach using a universal pH indicator to differentiate inorganic anions according to their relative acidity or basicity is presented. Anions caused changes in the pH of the solution, producing various colors of the universal indicator. Among halides, F- was differentiated from Cl-, Br- and I-. The indicator was also used on conjugate acid-base pair anions to distinguish HCO3- from CO32-, HSO4- from SO42-, HSO3- from SO32-, and various phosphate species. Oxyanions SO32-, HSO3-, ClO-, ClO2- and NO2- can be differentiated from oxyanions with more oxygens attached, namely, SO42-, HSO4-, ClO3-, and NO3-, respectively. Results can be correlated with the acid ionization constant Ka and/or base hydrolysis constant Kb of the anion.
KEYWORDS:Anion Differentiation; Colorimetric; Ionization Constants; pH; Universal pH Indicator
Download this article as:| Copy the following to cite this article: Rodrigo J. G. K, Organo V. G. Universal pH Indicator as a Colorimetric Reagent for Differentiating Inorganic Anions. Orient J Chem 2020;36(3). |
| Copy the following to cite this URL: Rodrigo J. G. K, Organo V. G. Universal pH Indicator as a Colorimetric Reagent for Differentiating Inorganic Anions. Orient J Chem 2020;36(3). Available from: https://bit.ly/2YHXanQ |
Introduction
Acid-base indicators or pH indicators are a class of dyes that are common in most chemistry laboratories. These are weak acids or bases of which its undissociated form exhibits a color different from its ionic form.1 It has been developed, primarily, for determining the pH of solutions. However, its application has expanded to include sensing various analytes such as gases,2–7 organic compounds,8,9 and cations.10,11
Recently, our group has shown that anions can be differentiated based on its acidic or basic properties. Using flower pigments12 or common laboratory pH indicators,13 different anions change the color of the indicator depending on the pH produced in solution. This approach has been applied to differentiate conjugate acids and bases such as carbonates, sulfates and phosphates. While flower pigments can be extracted easily from natural sources, its color was found to be unstable for long periods of time and tend to vary depending on the extraction procedure and its source. Common laboratory pH indicators, on the other hand, are more stable and are readily available. However, the pH range of individual indicators is limited; thus, requiring several indicator solutions to perform the analysis.
In this study, a universal pH indicator serves as a “single” colorimetric reagent for the qualitative profiling of anions. This approach is simple, easy to conduct, and requires small amounts of reagents for analysis. The method can have practical applications in chemical education. It can serve as a teaching demonstration or microscale laboratory experiment in senior high school or undergraduate general chemistry, or analytical chemistry class to illustrate solution properties of anions, understand the nature of amphiprotic anions, and relate the effect of equilibrium constants Ka and Kb to varying anionic species.
Materials and Methods
Materials and Equipment
Analytical grade sodium and potassium salts used in this study, namely NaF, NaI, Na2SO4, NaHSO4, NaHSO3, Na2SO3, NaClO, NaClO2, NaNO3, NaNO2, Na3PO4, Na2HPO4, NaH2PO4, KCl, KBr, KHSO4, and KClO3 were purchased from commercial sources and used without further purification. The universal pH indicator (pH 4-10) was purchased from Sigma-Aldrich™.
Reagent and Microplate Colorimetry Preparations
1.5 mL of 0.1M salt solutions in distilled or deionized water were prepared. 0.3 mL of each solution were placed into 96-well microplates, with one column composed of three wells serving as the three trials for each salt. A separate column was used for the control group (water only). 15.6 mL of the universal pH indicator was added into each test solution.
Results
One of the most versatile acid-base indicators developed is the universal pH indicator.14 Also known as Yamada Universal Indicator, this solution consists of thymol blue, bromothymol blue, phenolphthalein and methyl red.15 It operates at long pH ranges (pH 4-10). In this study, a universal pH indicator was used as a “single” reagent for differentiating anions. The addition of the universal pH indicator to individual solutions of anions led to color changes as shown in Fig. 1.
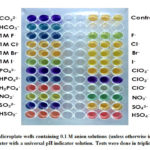 |
Figure 1: Microplate wells containing 0.1 M anion solutions (unless otherwise indicated) in distilled water |
Among the halides, F–, Cl-, Br–, and I–, only F– was generally distinct from the others. F– led to an olive-green solution while other halides have colors similar to the control solution at 0.1M concentration. At a higher concentration (1M), F– is still significantly different from Cl–, Br–, and I–, but the color has turned to blue. Moreover, I– could now be distinguished from the other halides with a color change from yellow to olive-green. This observation may be attributed to an increase in ionic strength of halide solutions which caused a shift in the color of the indicator. This behavior has also been noted by Rodriguez and Mirenda16 in which an increase in salt concentration led to a shift of the pH indicator towards the basic form. Thus, it is important to compare anions with the same concentration and under dilute conditions.
Conjugate acids/bases were also distinguished from each other. It was observed that the color of the SO42- solution is yellow while that of HSO4– is red. Meanwhile, SO32- was notably violet while HSO3– was red-orange. Different phosphate species exhibited different colors: PO43- is violet, HPO42- is blue, and H2PO4– is orange. It was also observed that CO32- is violet while HCO3– is blue. As expected, the conjugate acid form of the anion tends to shift the color of the indicator towards the acidic color relative to its basic color.
Finally, oxyanion pairs sulfate-sulfite, bisulfate-bisulfite, nitrate-nitrite, and oxychlorides were also differentiated from each other. Less-oxygenated oxyanions SO32- and ClO2– resulted in blue to violet colors. ClO–, at the onset, is violet but eventually turns to light green, then colorless. It should be noted that hypochlorite, ClO–, is a widely-used bleach and a strong oxidant, and is known to degrade dyes.17,18 Meanwhile, oxyanions with a higher number of oxygen atoms attached, SO42- and ClO3–, result in a yellow solution. On the other hand, for oxyanions NO3– and NO2–, nitrate exhibited a dark green color, while nitrite ion yielded a yellow-green solution.
The analysis was also performed in degassed distilled and degassed deionized water. Similar results were obtained as those for simple distilled water. This implies that minimal impurities like dissolved gases in the air do not significantly affect the results of the analysis.
Discussion
The colors observed for anion solutions correlate well to the colors of the indicator at a given pH of the solution. This was verified using a pH meter. Conjugate bases and less- oxygenated oxychlorides ClO2–, ClO–, CO32- and PO43- with pH of 10 or greater exhibited a violet color. SO32- and HPO42- with pH 9 to less than 10 imparted a blue solution. HCO3– with pH ranging from 8 to less than 9 yielded a blue-green color. On the other hand, NO3– and F– with pH 7 to less than 8 presented a green color. Oxyanions SO42-,ClO3– and NO2– with pH from 6.4 to less than 7 displayed a yellow-green solution. Meanwhile, halides I–, Cl–, and Br– with pH from 6 to less than 6.4 imparted a yellow color. For protonated anions, H2PO4– (pH 4-5) gave off orange color, while HSO3– and HSO4– (pH < 4) yielded red-orange or red, respectively.
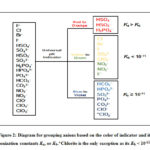 |
Figure 2: Diagram for grouping anions based on the color of indicator |
Aside from pH, the color of the solution imparted by anions can be correlated to its acid ionization constant (Ka) or base hydrolysis constant (Kb).19 This allows grouping of anions based on its acidic or basic properties as shown in Figure 2. Anions which turn the pH indicator to red or red-orange (HSO4–, HSO3– and H2PO4–) are protonated species and were found to have Ka values greater than its Kb (Table 1). Thus, these anions tend to produce more H3O+ in solution via acid ionization than OH– ions produced via base hydrolysis, as exemplified by HSO4– in Fig. 3.
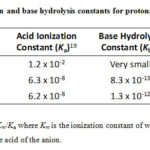 |
Table 1: Acid ionization and base hydrolysis constants for protonated anions |
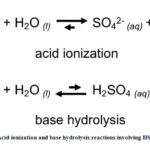 |
Figure 3: Acid ionization and base hydrolysis reactions involving HSO4-. |
On the other hand, anions that turn the pH indicator to yellow or yellow-green (Cl–, Br–, I–, SO42-, NO2– and ClO3–) or green (F– and NO3–) were found to have Kb values less than 10-11. Finally, anions that turn the pH indicator to blue or violet (ClO2–, SO32-, HPO4–, PO43-, HCO3–, CO32- and ClO–) are relatively basic. These anions, except for ClO2– (Kb = 8×10-13), were found to have Kb values greater than or equal to 10-11.
Conclusion
A universal pH indicator solution was used to differentiate inorganic anions. The indicator was able to differentiate F– from the other halides. Protonated anions (HSO4–, HSO3– and H2PO4–) were also differentiated from its conjugate base pairs (SO42-, SO32-, HPO42- and PO43-). Oxyanions SO32-, HSO3–, NO2–, ClO– and ClO2– were differentiated from oxyanions with more oxygen atoms attached, namely SO42-, HSO4–, NO3– and ClO3–. The color produced can be correlated with the pH of the solution and corresponding acid ionization constant Ka and/or base hydrolysis constant Kb of the anion.
This approach is simple and can be used as a demonstration or laboratory experiment in high school or freshmen college chemistry to help learners understand the acidic or basic nature of anions. It also applies principles of chemical equilibrium, ionization constants, and nature of amphiprotic anions through visual observation.
Acknowledgment
This research work was conducted under the Research Grants Administration Office (RGAO-2016-0835) of the University of the Philippines Manila.
Conflict of Interest
No conflict of interest.
References
- Sabnis, R. W. Handbook of Acid-Base Indicators; CRC Press, 2007.
- Solomon, S.; Oliver-Hoyo, M.; Hur, C. Generating Water-Soluble Noxious Gases: An Overhead Projector Demonstration. Chem. Educ. 1998, 75 (12), 1581–1582.
- Carvalho, A. P.; Mendonça, Å. F. S. S.; Piedade, M. F. M. Acid-Base Reactions with Carbon Dioxide. Chem. Educ. 2002, 79 (12), 1464A.
- Mills, A.; Skinner, G. A.; Grosshans, P. Intelligent Pigments and Plastics for CO2 J. Mater. Chem. 2010, 20, 5008–5010.
- Rodríguez, A. J.; Zamarreño, C. R.; Matías, I. R.; Arregui, F. J.; Cruz, R. F. D.; May-Arrioja, D. A. A Fiber Optic Ammonia Sensor Using a Universal pH Indicator. Sensors (Switzerland) 2014, 14 (3), 4060–4073.
- Feng, L.; Musto, C. J.; Kemling, J. W.; Lim, S. H.; Zhong, W.; Suslick, K. S. Colorimetric Sensor Array for Determination and Identification of Toxic Industrial Chemicals. Chem. 2010, 82 (22), 9433–9440.
- Feng, L.; Musto, C. J.; Kemling, J. W.; Lim, S. H.; Suslick, K. S. A Colorimetric Sensor Array for Identification of Toxic Gases below Permissible Exposure Limits. Commun. 2010, 46, 2037-2039.
- Lim, S. H.; Musto, C. J.; Park, E.; Zhong, W.; Suslick, K. S. A Colorimetric Sensor Array for Detection and Identification of Sugars. Lett. 2008, 10 (20), 4405–4408.
- Zhang, C.; Suslick, K. S. A Colorimetric Sensor Array for Organics in Water. Am. Chem. Soc. 2005, 127 (33), 11548–11549.
- Gokulan, G.; Nagarajan, D.; Venkatanarasimhan, S.; Sathish, A. Ixora Coccinea Floral Extract Coated Ear Buds – Highly Selective and Eco-Friendly Sensor for the Detection of Pb(II) Ions. Environ. Chem. Eng. 2019, 7 (4), 103230.
- Porrawatkul, P.; Pimsen, R.; Kuyyogsuy, A.; Nuengmatcha, P. Simple and Selective Naked-Eye Detection of Cu2+ and Al3+ using Hibiscus rosa-sinensis Linn Flower Extract. J. Chem. 2018, 34 (1), 188–195.
- Galingana, M. O.; Organo, V. G. A Simple Colorimetric Procedure for Differentiating Anions Using Flower Pigments from Anthurium andreanum. J. Chem. 2016, 32 (3), 1347–1352.
- Punzalan, J. M.; Organo, V. G. Are Aqueous Solutions of Amphiprotic Anions Acidic, Basic, or Neutral? A Demonstration with Common pH Indicators. Chem. Educ. 2017, 94 (7), 911–915.
- Myers, R. J. One-Hundred Years of PH. Chem. Educ. 2010, 87 (1), 30–32.
- Foster, L. S.; Gruntfest, I. J. Demonstration Experiments Using Universal Indicators. Chem. Educ. 1937, 14 (6), 274–275.
- Rodríguez, H. B.; Mirenda, M. A Simplified Undergraduate Laboratory Experiment to Evaluate the Effect of the Ionic Strength on the Equilibrium Concentration Quotient of the Bromcresol Green Dye. Chem. Educ. 2012, 89 (9), 1201–1204.
- Oakes, J.; Gratton, P.; Gordon-Smith, T. Combined Kinetic and Spectroscopic Study of Oxidation of Azo Dyes in Surfactant Solutions by Hypochlorite. Dyes and Pigments 2000, 46 (3), 169–180.
- Oakes, J.; Gratton, P. Kinetic Investigations of Azo Dye Oxidation in Aqueous Media. Chem. Soc. Perkin Trans. 2 1998, 9, 1857–1864.
- Perrin, D. D. Dissociation Constants of Inorganic Acids and Bases in Aqueous Solution. Pure Appl. Chem. 1969, 20 (2), 133–236.

This work is licensed under a Creative Commons Attribution 4.0 International License.










