Synthesis, Characterization, Antibacterial, Antifungal, Antioxidant and Anti-Inflammatory Actions of Novel β-Diketone Complex
Sachin Govind Bibave1* and Anil E. Athare2
1Agasti Arts, Commerce And Dadasaheb Rupawate Science College Akole. Tal Akole, Dist Ahmednagar. Maharashtra, India.
2Department of Chemistry, New Arts, Commerce And Science College Ahmednagar. Maharashtra, India.
DOI : http://dx.doi.org/10.13005/ojc/350624
Article Received on : 08-11-2019
Article Accepted on : 07-12-2019
Article Published : 20 Dec 2019
β-diketone (βd) is prepared using the name reaction Baker-Venkataraman transformation reaction. In this present work, ester (c’) was prepared when 1-(2-Hydroxy-5-methyl-phenyl)-ethanone (a’) was treated with 4-Propoxy-benzoic acid (b’) at 0 °C to 10 °C. (c’) on Baker-Venkataraman transformation to give β-diketone ligand (L’) named as 1-(2-Hydroxy-5-methyl-phenyl)-3-(4-propoxy-phenyl)-propane-1,3-dione. Bidentate ligand (L’) treated with Cu (II) nitrate gives Cu (II) complex (CuL’). (L’) shows tautomerism, this tautomerism phenomenon was studied using FTIR and NMR. Because of enol serve as ligand (L’) in the synthesis of (CuL’). The prepared (L’) can be characterized by HR-MS, elemental analysis, 1H, 13C, DEPT, D2O exchange, FTIR. The antibacterial, antifungal, antioxidant, anti-inflammatory property of (CuL’) is studied.
KEYWORDS:Baker-Venkataraman transformation, β-diketone ligand, Metal complex, Antioxidant activity, Anti-inflammatory Activity, Antibacterial activity, Antifungal agent.
Download this article as:| Copy the following to cite this article: Bibave S. G, Athare A. E. Synthesis, Characterization, Antibacterial, Antifungal, Antioxidant and Anti-Inflammatory Actions of Novel β-Diketone Complex. Orient J Chem 2019; 35(6). |
| Copy the following to cite this URL: Bibave S. G, Athare A. E. Synthesis, Characterization, Antibacterial, Antifungal, Antioxidant and Anti-Inflammatory Actions of Novel β-Diketone Complex. Orient J Chem 2019; 35(6). Available from: https://bit.ly/36UzPBH |
Introduction
Transition elements plays an important role in the formation of chemical complex of co-ordination chemistry. Massive literature is available on the application of complexes. (βd) and it’s metal complexes play an important role in co-ordination chemistry1. As starting material in the formation of new material or in the thin film for optical device applications2, radiopharmaceuticals3. (βd) acts as multipurpose precursor for many reactions of numerous heterocycles4 for example flavones5, isoxazolyl, pyrazolic6, pyrimidines, triazolic7. (βd) is used in metal withdrawal by chelating agent8, textile9, resin10, sunscreen cosmetics11, HIV-1 avoidance12. Ln (III) (βd) shows luminescent property13. (βd) hydrolase shows enzymatic activities14. (βd) with different attachments and its relative complexes being Lewis acerbity, volatility, ideal molar heat of sublimation, ideal molar enthalpy of formation, steam pressures, catalytic property calculated15. (βd) shows pharmacological property such as antimicrobial, anticancer16, antibacterial, antifungal, antioxidant17, anti-inflammatory18. (βd) is found to be dynamic against anti-influenza19. (βd) also used in new advanced energy conserving lighting technology i.e. organic light emitting diodes (OLED)20. (βd) show inside similar molecule hydrogen link keto and enol tautomerization21. Considering all above properties, in this paper, include (L’) as a (βd) and it’s complex (CuL’). synthesized (L’) and complex (CuL’) are characterized by analytical techniques and they are used to test antibacterial, antifungal, antioxidant and anti-inflammatory properties.
Experimental
All analytical quality chemicals are utilized for the analysis work and they are bought from the authorized chemical merchant. Chemicals are used without additional purification. The standard procedure uses to purify Solvents. Super dry distilled pure ethanol used for recrystallization and preparation of complex.
Synthesis of 4-Propoxy-benzoic acid 2-acetyl-4-methyl-phenyl ester (c’)
At room temperature, equimolar 1-(2-Hydroxy-5-methyl-phenyl)-ethanone and 4-Propoxy-benzoic acid is dissolved in solvent pyridine and stirred for 10 min using magnetic stirrer. Then to maintain the temperature of the reaction mixture to 0 °C. In this mixture POCl3 added drop by drop with constant stirring, the temperature of the reaction mixture maintains below 10°C. After complete addition of POCl3, the mixture blend for 3 hrs. with the help of magnetic stirrer. The growth of process is tested by T L Chromatography and then the blend is kept whole night at normal temperature. Then the blend is added slowly on small mashed ice and if necessary acidulent using 1M HCl. The output of the reaction is filtered and it is washed using water and the creation is re-purified using ethyl alcohol. The purity of the compound is tested by TLC. The yield of the product (c’) is 81%. m. p. 94°C.
Synthesis of β-diketone (L’)
4-Propoxy-benzoic acid 2-acetyl-4-methyl-phenyl ester (c’) (3.121 g , 0.01 mol ) is dissolved in (15 ml) pyridine and in this mixture pulverized powder KOH (1.122 g , 0.02 mol ) is combined. The mixture is blend for 3 hours and the growth of process checked by T L C. Afterward the accomplishment of the process, the mixture is spread on mashed ice and it is acidify through 1 M conc. HCl. Yellow solid of (L’) is obtained. It is filtered by using Buchner funnel. The product is recrystallized using ethanol. The purity of the compound is tested by TLC. The yield of the product is 71%, m. p. 190°C.
Synthesis of metal complex (CuL’)
The complex is made in metal to the ligand molar ratio 1:2 respectively. In hot alcoholic solution of (L’), hot alcoholic metal nitrate is combined dropwise. The mixture is blend and reflux for 3-4 hrs. as a result precipitation of complex takes place afterward the combination of alc. NH3. The product is then filtered and washed by using ethyl alcohol and dry. The colour of (CuL’) is green. Yield 68%, m.p.: 290 °C, elemental analysis: calculated C, 66.51; H, 5.58; O, 18.65; Cu, 9.26; found C, 66.57; H, 5.96; Cu, 9.40.
 |
Plan 1 Click here to View Figure |
Characterization
Melting points find out using Thiel’s tube, glass capillary method. The elemental analysis is accomplished using FLASH EA 1112 series Thermo Finnigan. FT-NMR using Bruker AV. II., 1H frequency is 400 MHz and 13C frequency is 100MHz with the help of T M S internal std. and CDCl3 as sol. FTIR reported on FTIR Spectrophotometer make Bruker Optics and model ALPHA-T. The sample is placed on ATR crystal. UV-Visible tested on Perkin Elmer. Conductance is reported on Equip-Tronic conductivity meter. The magnetic susceptibility is tested via Guoy balance. Physical properties of the ligand and complex is studied. The analytical data metal to ligand molar ratio of the complex is 1:2. Absence of coordinated water molecules in tetra-coordinated metal complex, due to this the probable geometry is tetrahedral.
1H, 13C NMR, HR-Mass statistics of ligand (L’)
1H, 13C FT-NMR of the ligand are tested using CDCl3 solvent with the projected skeleton. 1H FT ̶ NMR, 400 MHz, CDCl3 δ: signal of methyl group linked to -CH2-CH2-O- group at 1.058 (t, 3H, ̶ CH3), 1.833 (m, 2H , ̶ CH2), signal of methyl group linked to benzene ring at 2.327 (s, 3H, ̶ CH3), 3.984(t, 2H, ̶ CH2), Signal of ̶ CH= group linked to carbonyl group at 6.733 (s, 1H, ̶ CH=), signals of aromatic H appeared at 6.878-7.977 (m, 7H, Ar ̶ H), signal of phenolic-OH at 11.955 (s, 1H, Ar ̶ OH), signals of enolic -OH at 15.846 (s, 1H, enolic ̶ OH), 13C FT ̶ NMR 100 MHz, CDCl3 δ: Signal of carbonyl carbon at 194.73 (s, C ̶ 1, >C=O), 90.92 (s, C ̶ 2, >CH=), 177.76 (s, C ̶ 3, >C ̶ OH), 128.04 (s, C ̶ 1’), 128.82 (s, C ̶ 2’), 114.45 (s, C ̶ 3’), 162.84 (s, C ̶ 4’), 114.58 (s, C ̶ 5’), 128.07 (s, C ̶ 6’), 69.76 (t, C ̶ 7’), 22.46 (m, C ̶ 8’), 10.47 (t, C ̶ 9’), 125.60 (s, C ̶ 1’’), 131.22 (s, C ̶ 2’’), 119 (s, C ̶ 3’’), 136.52 (s, C ̶ 4’’), 118.68 (s, C ̶ 5’’), 160.16 (s, C ̶ 6’’), 20.59 (s, C ̶ 7’’), DEPT 135 use to confirm CH, CH3 and CH2 in ligand. Total 10 CH, CH3 and 2 CH2 presents in ligand are confirmed by DEPT 135. D2O Exchange uses to confirm enolic-OH, Phenolic-OH functional group proton. HR-MS: 313.1438 (M+H), 335.1258 (M+Na).
 |
Figure 1: HR-MS of (L’) Click here to View Figure |
Magnetic Susceptibility
The magnetic moment of (CuL’) is measured at room temperature for one unpaired electron is 2.22 B.M which is somewhat higher than the (1.73B.M) spin only value for unpaired one electron, which can be peculiar magnetic moment and orbit spin coupling nearer to spin only value generally of square pyramidal type.
Molar conductance
Molar conductivity of (CuL’) is measured in DMSO at concentration of 10-4 M. Molar conductivity of (CuL’) is 25.77 Ω-1 cm2 mol-1 which indicates that (CuL’) is non-electrolyte and covalent in nature.
FT-IR Spectra
The FT ̶ IR spectrum of ligand and its metal complex is recorded and equated. The (L’) ligand entirely enolized in ethanol, ν(˃C=O) of ligand 1624.3 cm-1. ν ( ̶ C=C ̶ ) 1582.7 cm-1 and ν(C ̶ O) at 1198.9 cm-1. In metal complex IR frequency of carbonyl ν(˃C=O) 1608.5 cm-1 which is lower than IR frequency of (L’) 1624.3 cm-1 22. This lowering frequency of complex shows that (L’) coordinated with Cu metal ion. New band at 532 cm-1 due to (M-O) metal to oxygen bond in metal complex. This proves that metal co-ordinates with (L’) through oxygen. Absence of peak between 3547-3407cm-1 confirm that the absence of coordinated water in ligand and in complex23. The FT ̶ IR spectrum of the (L’) and (CuL’) are comparable apart from little minor variation in vibration band produced via metal ion.
Electronic absorption Spectra of (CuL’)
The UV spectrum of (CuL’) taken in DMSO as a solvent. The observed wave numbers (ν) are 26455.03 cm-1, 32786.89cm-1, 40650.41cm-1, 45662.10cm-1 , 46296cm-1. The band range observed between 31000-38000 cm-1 also metal to ligand charge transfer band between 25000-26000cm-1 obscures the d-d transition shows square pyramidal geometry.
Thermogravimetric investigation of metal [Cu(L’)2] complex
Thermal decomposition of selected metal complex was carried out at a heat amount of 10°C min-1 using N2 environment between the thermal scale 32.64 to 1000°C in N2 environment. The thermogravimetric spectrum of [Cu(L’)2] expresses no mass fall between the temperature range 32.64 °C to 200 °C which clearly shows the absence of lattice and coordinated water molecules in [Cu(L’)2]. The (CuL’) gives disintegration from thermo scale 200°C to 400 °C, by obs. 61.83%, cal. 62.78% mass fall due to elimination of the (C27H26O5) part of (L’), the endothermic process (TDTA) peak at 290°C, which assigned to melting point [melting point determined by capillary tube system in static air was (290 oC)]. The subsequent disintegration phase starts after 450°C to 540°C by mass fall of obs. 25.95%, cal. 25.69% resembles to the disintegration of the link (C11H12O2) portion of the (L’). An exothermic peak near thermo scale from 508°C in the DTA seen for this phase. On further heating up to 938.81 °C, the remaining weight corresponds to that of copper oxide (CuO).
 |
Figure 2: DTA/TGA Curves of [Cu(L’)2] Click here to View Figure |
Antimicrobial Activity
Antimicrobial activity of (L’) and (CuL’) was determined against Gram-negative Escherichia coli and another Gram-positive Bacillus subtilis class by well diffusion method. Antifungal activity was determined against Aspergillus niger by well diffusion method. The samples were prepared by dissolving them into DMSO at concentration of 10 mg/ml.
Antibacterial Property
(L’) does not show any antibacterial against E. coli and B. subtilis. The positive control is antibiotic streptomycin and shows region of reserve of 6 mm and 4mm in contradiction of E. coli and B. subtilis respectively. Negative control contains DMSO does not show any antibacterial activity. (CuL’) does not show any antibacterial in contradiction of E. coli and B. subtilis. The positive control is antibiotic Chloramphenicol and shows region of reserve of 7 mm and 9 mm in contradiction of E. coli and B. subtilis respectively. Negative control contains DMSO does not show any antibacterial activity.
Antifungal Property
(L’) does not show any antifungal activity against A. niger. Positive control for antifungal activity is clotrimazole and shows zone of inhibition of 7 mm. Negative control contains DMSO does not show any antimicrobial activity. (CuL’) does not show any antifungal activity against A. niger. Positive control for antifungal activity is clotrimazole and shows zone of inhibition of 7 mm. Negative control contains DMSO does not express antimicrobial activity.
Table 1: Antimicrobial Action of Ligand and its Metal Complex
| S.N. | Compounds | Antibacterial activity | Antifungal activity | ||||
| Zone of reserve (mm) | Zone of reserve (mm) | ||||||
|
|||||||
| +ve Control | -ve Control | +ve Control | -ve Control | +veControl | -ve
Control |
||
| 1 | (L’) | ̶ | ̶ | ̶ | ̶ | ̶ | ̶ |
| 2 | Streptomycin | 6 | ̶ | 4 | ̶ | ̶ | ̶ |
| 3. | Clotrimazole | ̶ | ̶ | ̶ | ̶ | 7 | _ |
| 4. | (CuL’) | ̶ | ̶ | ̶ | ̶ | ̶ | ̶ |
| 5. | Chloramphenicol | 7 | ̶ | 9 | ̶ | ̶ | ̶ |
| 6. | Clotrimazole | ̶ | ̶ | ̶ | ̶ | 7 | ̶ |
Antioxidant activity
Antioxidant activity of (CuL’) checked against the standard as ascorbic acid.
Table 2: Percent inhibition and IC50 values
|
Compounds |
% inhibition at Concentration(µg/ml) | IC50µg/ml | ||||
| 200 | 400 | 600 | 800 | 1000 | ||
| (CuL’) | 22.00 | 32.11 | 44.67 | 59.78 | 68.33 | 644.74 |
| Ascorbic acid | 52.55 | 65.41 | 76.32 | 86.95 | 95.25 | 126.84 |
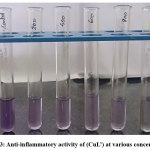 |
Figure 3: Anti-inflammatory activity of (CuL’) at various concentration Click here to View Figure |
For synthesized chemical compounds, the IC50 value 644.74 µg/ml is greater than standard ascorbic acid value 126.84 µg/ml. Thus, the synthesized (CuL’) complex shows antioxidant activity.
Anti-inflammatory activity
The anti-inflammatory activity of compound (CuL’) was determined by HRBC membrane stabilization method. Blood was collected from healthy volunteers. The collected blood was mixed with equal volume of (2% dextrose, 0.8% sodium citrate, 0.05% citric acid & 0.42% sodium chloride in water). The blood was centrifuged at 3000 rpm and packed cells were washed with iso-saline (0.85%, pH 7.2) & 10% v/v suspension was made with iso-saline. The assay mixture contained the drug (at various concentration as 100-500µg). 1 mL phosphate buffer (0.15 M, pH 7.4), 2 mL of hypo-saline (0.36%) and 0.5 mL of HRBC suspension. Diclofenac was used as the reference drug. Instead of sample, same volume of distilled water was used as control. All the assay mixtures were incubated at 37˚C for 30 min and centrifuged. The hemoglobin content in the supernatant solution was estimated using colorimeter at 560 nm. The percentage of HRBC membrane stabilization or protection was calculated using the following formula:
% Protection against hemolysis = 100 – (O.D. of test sample / O.D. of control) × 100
Observation and Result
Table 3: Absorbance and percent HRBC membrane protection.
| Sr. No. | Concentration | Absorbance at 560 nm | % Protection | ||
| Diclofenac | Sample | Diclofenac | Sample | ||
| 1 | 0 (Control) | 0.925 | 0.925 | 0.00 | 0.00 |
| 2 | 100 | 0.731 | 0.813 | 20.97 | 12.10 |
| 3 | 200 | 0.703 | 0.781 | 24.00 | 15.56 |
| 4 | 300 | 0.678 | 0.749 | 26.70 | 19.02 |
| 5 | 400 | 0.645 | 0.716 | 30.27 | 22.59 |
| 6 | 500 | 0.611 | 0.685 | 33.94 | 25.94 |
The concentration range from 100 μg/mL to 500 μg/mL protects the human erythrocyte membranes against lysis induced by hypotonic solution. At concentration of 500 μg/mL, the sample was inhibited 25.94% of RBC haemolysis as compared with 33.94% produced by Diclofenac at 500 μg/mL. The results obtained demonstrated that sample can significantly and dose dependently inhibit HRBC haemolysis.
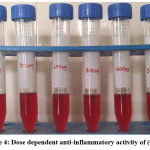 |
Figure 4: Dose dependent anti-inflammatory activity of (CuL’). Click here to View Figure |
Conclusion
(L’) prepared via Baker-Venkataraman transformation of (c’). It’s transition metal complex (CuL’) is created and defined by analytical statistics, spectroscopic statistics and physical statistics. The (L’) perform bidentate ligand and approach to d-block metal like Cu (II). The (CuL’) has another representation formula [Cu(L’)2] where (L’) means 1,3-βdiketone ligand and it’s metal complex is studied for antioxidant, anti-inflammatory, antibacterial, antifungal agent. The biotic action statistics gives (L’) and (CuL’) inhibit antibacterial, antifungal movement contrary to different bacteriological and fungiform species. (CuL’) shows antioxidant and anti-inflammatory action.
Acknowledgements
The author is thankful of the Principal & HOD of Dept. of Chem. New Arts, Comm. And Sci. College Ahmednagar, Maharashtra and Principal, Agasti Arts, Commerce & Dadasaheb Rupawate Science College, Akole, Tal Akole, Dist Ahmednagar, Maharashtra, India for allowing the lab. facility.
Conflicts of Interest
Authors do not have any conflict of interest.
References
- Garnovskii, A.D.; Kharisov, B.I.; Blanco, L.M.; Garnovskii, D.A.; Burlov, A.S.; Vasilchenko, I.S.; Bondarenko, G.I. J. Coord. Chem., 1999, 46(4), 365-395.
- Gilberto, F.d.; Alves, S.; Silva, B.J.P.; Silva, E.F. Opti. Mate., 1998, 11(1), 23-28.
- Clark, S.; Elliott, J.M.; Chipperfield, J.R.; Styring, P.; Sinn E. Inorg. Chem. Commu., 2002, 5(4), 249-251.
- Surati, K.R. Spectro. acta. Part A, Mole. biomole spectro., 2011, 79(1), 272-277.
- Chee, C.F.; Buckle, M.J.C.; Rahman, N.A. Tetrahedron Lette., 2011, 52(4), 3120-3123.
- Marinnzik, A.L.; Felder, E.R.; Tetra. Lette., 1996, 37(7), 1003-1006.
- Shaabani, A.; Rahmati, A.; Rezayan, A.H.; Darvishi, M.; Badri, Z.; Sarvari, A. Molec. Infor., 2007, 26(9), 973-979.
- Scribner, W.G.; Smith, B.H.; Moshier, R.W.; Sievers, R.E. J. Org. Chem., 1970, 35(5), 1696-1698.
- Saenz, M.; Alvarado, J.; Pereira, F.P.; Ferreiro, S.S.; Lavilla, I.; Bendicho, C. Ana. Chimi. Acta, 2011, 687(1), 50-55.
- Kumar, R.; Jain, S.K.; Misra, R.K.; Kachchwaha, M.; Khatri, P. K. Inter. J. Envir. Sci. and Tech., 2012, 9(1), 79-84.
- Paris, C.; Vallet, V.L.; Jiménez, O.; Trullas, C.; Miranda, M.Á. Photochem. and Photobio., 2009, 85(1), 178-184.
- Tchertanov, L.; Mouscadet, F. J. Medi. Chem.,2007, 50(6), 1133-1145.
- Bellusci, A.; Barberio, G.; Crispini, A.; Ghedini, M.; Deda, M.L.; Pucci, D. Inorg. Chem., 2005, 44(6), 1818-1825.
- Mougin, C.; Boyer, F.D.; Caminade, E.; Rama, R. J. Agri. Food Chem., 2000, 48(10) 4529-4534.
- Manuel, A.V.R.D. Silva.; Luis, M.N.B.F. Santos. The J. Chemic. Thermo., 2006, 38(7), 817-824.
- Shehab, W.S.; Bassyouni, G.T.E. J. Iranian Chemic. Soc.,2018, 15(7), 1639-1645.
- Sheikh, J.; Parvez, A.; Juneja, H.; Ingle, V.; Chohan, Z.; Youssoufi, M.; Hadda, T.B. Europ. J. medici. Chem., 2011, 46(4), 1390-1399.
- Aggarwal, R.; Kumar, S.; Kaushik, P.; Kaushik, D.; Gupta, G.K.; Europ. J.medici. chem., 2013, 62, 508-514.
- Abdella, A.M.; Moatasim, Y.; Ali, M.A.; Elwahy, A.H.M.; Abdelhamid, I.A. J. Heterocyc. Chem., 2017, 54(3) 1854-1862.
- Kalyani, N.T.; Dhoble, S.J. Renew. Sustain. Ener. Revi., 2012,16(5), 2696-2723.
- Wallen, S.L.; Yonker, C.R.; Phelps, C.L.; Wai, C.M. J. Chem. Soc., Faraday Transac.,1997, 93, 2391-2394.
- Gren, E.Y.; Grinvalde, A.K.; Vanag, G.Y. J. Appli. Spectr., 1967, 6(3), 253-255.
- Chen, Z.; Wu, Y.; Huang, F.; Gu, D.; Gan, F. Spectrochimi. acta. Part A, 2007, 66(4-5), 1024-1029.

This work is licensed under a Creative Commons Attribution 4.0 International License.









