Facile Synthesis, Characterization and Antimicrobial Activities of Novel 6-AminoTriazolo-Thiadiazoles Integrated with Benzofuran and Pyrazole Moieties
Mohammad Idrees1*, Satish S. Kola1, Naqui J. Siddiqui2
1Department of Chemistry, Institute of Science, Nagpur (M.S.), India
2Department of Chemistry, Government Science College, Gadchiroli (M.S.), India
Corresponding Author Email: idreesshaikh.2009@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350612
Article Received on : 07-09-19
Article Accepted on : 05-12-2019
Article Published : 25 Dec 2019
In the undertaken research we have reported simple efficient technique to afford a novel series of 3-(5-(benzofuran-2-yl)-1-phenyl-1H-pyrazol-3-yl)-N-aryl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-amine (4a-g) derivatives obtained by one pot cyclocondensation reaction of 5-(5-(benzofuran-2-yl)-1-phenyl-1H-pyrazol-3-yl)-4-amino-4H-1,2,4-triazole-3-thiol (3) with substituted aryl isothiocyanate in DMF and K2CO3, without formation undesirable side products by simple work up procedure. The structures yielded (4a-g) were established by 13CNMR, IR, 1HNMR, elemental analysis and mass spectra. Entire synthesised compounds were screened for their in-vitro biological assay via microorganism Gram positive and Gram negative bacterial strains at different concentrations. The bioassay revealed that some of the compounds have promising antimicrobial activities when compared with standard drug Chloramphenicol.
KEYWORDS:Antimicrobial; Benzofuran-2-Yl; Pyrazole; Triazolothiadiazole
Download this article as:| Copy the following to cite this article: Idrees M, Kola S. S, Siddiqui N. J. Facile Synthesis, Characterization and Antimicrobial Activities of Novel 6-AminoTriazolo-Thiadiazoles Integrated with Benzofuran and Pyrazole Moieties. Orient J Chem 2019; 35(6). |
| Copy the following to cite this URL: Idrees M, Kola S. S, Siddiqui N. J. Facile Synthesis, Characterization and Antimicrobial Activities of Novel 6-AminoTriazolo-Thiadiazoles Integrated with Benzofuran and Pyrazole Moieties. Orient J Chem 2019; 35(6). Available from: https://bit.ly/2MtyUQT |
Introduction
In recent years, fused heterocycles with three hetero atoms in five membered aromatic structures such as 1,2,4-triazoles and thiadiazoles sizable devotion owing to synthetic and remarkable pharmacological activities. The amino triazolo-thiadiazole system considered as a cyclic crucial and versatile fused five membered heterocycle ring structure incorporating two nitrogen and one sulphur atom. The numbers of triazoles fused to thiadiazoles exhibit various therapeutically important property probably due to the existence of N-C-S fragment in ring. Literature survey has revealed that the 6-amino thiadiazole structure plays vital role in biologically active compounds consequently represents fascinating moiety for therapeutic chemistry.1,3,4-thiadiazoles are vital classes of azoles with significant pharmacological activities such antimicrobial1-6, antioxidant7, antituberculosis8, anticancer9-11, analgesic12,anti-inflammatory13, antiviral14-15, antifungal16-19, antitumor20, urease inhibitor21, analgesic and anti-inflammatory22, antidepressant23, anticonvulsant24, antimycotic25, diuretic26, cytotoxic27, corrosion inhibition effect28, antiproliferative29, anthelmintic 30. Moreover, nowadays researchers desire to yield fused or hybrids of various heteroatom ring to improve the medicinal property.
Prompted by these interpretations and in extension of our of determinations towards the synthesis of novel heterocyclic combinations with potent antimicrobial properties, in the present research we planned to frame a molecule and to synthesize and characterize a new series of condensed systems which combines two bio labile rings give a condensed and planar system of triazolothiadiazole with an anticipation to obtain compounds with better enhanced pharmacological activities and further thought of carrying out the antimicrobial studies of these innovative synthesized compounds against some bacterial strains.
Material and Methods
E. Merck TLC aluminium sheet silica gel was used for monitoring reactions; iodine and UV light chamber were utilized for visualizing of spots. Melting points obtained in open capillary tube. Shimadzu IR Spectrophotometer is used to record IR on a (KBr, ν max in cm-1). ESI mass spectra were noted on Waters Micromass Q–TOF Micro, Mass Spectrophotometer.1H NMR and 13CNMR spectra are logged in Bruker AM instrument having 400 MHz and values reported in (ppm) by (CDCl3 and DMSO-d6) in respect to tetramethylsilane (TMS). On Thermo Scientific (Flash-2000) element (CHN) analysis done using all the acquired products screened for their antimicrobial activities.
General protocol to yield 331
Suspension of potassium salt (2) (20 mmol), 95% hydrazine hydrate (40 mmol) in H2O (2mL) was taken in R.B flask and heated on condenser with occasional shaking for 1h. The colour of the content transformed to greenish with the evolution of H2S gas. The resultant solution was added to ice cold water. The solid 3 was separated out by acidification with conc. HCl, filtered, and recrystallization was carried out by using ethanol.
Synthesis of 6-amino-1,2,4-triazolo[3,4-b][1,3,4]thiadiazole (4a-h)
A mixture of compound (3, 3.74g,10mmol), and 1-bromo-4-isothiocyanatobenzene (2.14g, 10mmol) in DMF(25mL), was taken in round bottom flask to that potassium carbonate (1.38g,10mmol) was added then the reaction mixture was refluxed for 8h. Then reaction content was cooled discharged slowly with stirring into crush ice subsequent product obtained filtered, clean thoroughly with cold water. Correspondingly, 4b-g were synthesised from 3 by extending the same method followed for 4a and their structural identities were proved by chemical transformation reaction, physical data, and elemental analysis and IR spectra.
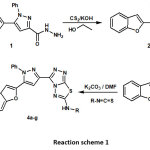 |
Scheme 1 Click here to View Scheme |
Spectral and Elemental Analysis
(4a): IR, 3440(N-H), 1594,1636(N-H), 3079,3020(CH ), 1497, 1518, 1594 (C=C),1255(C-O-C),1636(C=N), 1255( C-N), 999( N-N), 749(C-S-C) cm-1.1H NMR δ (ppm);14.86(s, 1H, aromatic secondary NH group), 6.59 (s, 1H, at C4 of pyrazole ring), 7.22-7.91(m, 14H, aromatic + Heteroaryl proton).13C NMR (DMSO-d6): δ (ppm)106, 111, 121, 123, 125, 127.41, 129, 135, 136, 138, 144(s, 1C, C3 of pyrazole ring), 153(s,1C, C9 of Benzofuran ring), 155, 161(s,1C,C2of triazolothiadiazole), 171(s, 1C, C8 of triazolothiadiazoles).GC-MS (m/z):556 [M+2]+.Elemental Analysis for C26H16BrN7OS Calculated, 56.33; H, 2.91; N, 17.68; S, 5.78 Found: C, 56.40; H, 2.98; N, 17.74; S, 5.70.
 |
Scheme 2 Click here to View Scheme |
(4b): IR, 3442(N-H), 1596, 1634(N-H), 3076,3018(C-H), 1495,1512,1596(C=C), 1256(C-O-C), 1634(C=N), 1256( C-N), 1002(N-N), 750(C-S-C) cm-1.
(4c): IR, 3441(N-H), 1591, 1638(N-H), 3077, 3023(C-H), 1496, 1516, 1598(C=C), 1259(C-O-C), 1634(C=N), 1259(C-N str.), 993(N-N), 742(C-S-C) cm-1.
(4d): IR, 3446(N-H), 3076, (C-H), 1497, 1594(C=C), 1257(C-O-C),1638(C=N), 1257(C-N), 994(N-N), 748(C-S-C) cm-1.
(4e): IR, 3442(N-H), 3079, 3025(C-H.), 2995(C-H), 2918, 2810(C-H), 1494, 1516, 1593(C=C), 1254(C-O-C), 1630(C=N), 1254(C-N),995(N-N), 753(C-S-C) cm-1.
(4f):IR, 3441(N-H), 3075,3021(C-H), 1375(C-H), 1499,1518,1593(C=C), 1256(C-O-C), 1078 (C-O-C), 1632(C=N),1256( C-N),996( N-N) ,745(C-S-C) cm-1.
(4g): IR, 3447(N-H), 3078, 3023(C-H), 1498, 1513, 1592 (C=C1254(C-O-C), 1634(C=N), 1254(C-N str.), 994(N-N), 756(C-S-C) cm-1.
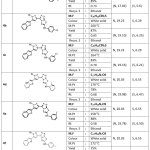 |
Table 1: Analytical and Physical data of 6-Amino Triazolothiadiazoles (4a-g) Click here to View Table |
Antimicrobial Activities: Procedure (cup plate agar disc-diffusion method)
Solutions of tested compounds (4a-g) were prepared by dissolving calculated amount of every yielded product in DMSO to give final concentration of 31-1000µg/mL. Petri plates and agar solution are sterilized in autoclave. Petri plate is arranged by pouring agar solution. Bacterial culture was inoculated on fresh nutrient broth and further diluted with water 0.1mL of diluted culture was banquet over nutrient agar in plate. Sterilized Whatmann paper circles (6mm) were soaked in different tested compoundsand dried at room temperature then applied on petri plate and incubated at 37°C for 24h zone of inhibition was measured in all direction in mm and taken as mean. Consequence was correlated with reference drug Chloramphenicol.
Result and Discussions
All novel synthesized compounds 4a-g has been corroborated by the spectroscopic examination such as FT-IR, 1HNMR, mass spectra and 13CNMR. For every final and intermediate product solubility and melting point was determined. The synthesis of target compound 6-amino triazolothiadiazole derivatives 4a-g was carried out by cyclocondensation reaction of compound (3) with aryl isothiocyanate in DMF and potassium carbonate. The synthetic protocol has been outline in scheme1.The FT-IR result of compounds 4a-g, revealed the disappearance of absorption bands due to -SH and -NH2 stretching frequencies of initial compounds 3 and appearance of broad band at 3440 cm-1 for NH stretch. The new band which appeared at 1636 cm-1 region is recognized to stretching frequency of C=N group of the thiadiazole ring further more absorption band at 749 cm-1 shows C-S-C stretch, undoubtedly indicated the cyclisation of compounds 3 and arylisothiocyanate in the presence of potassium carbonate to form triazolothiadiazole fused ring 4a.
1HNMR of 4a indicated disappearance of proton peak for –SH and –NH2 and occurrence of singlet at δ 14.56 ppm shows the existence of aromatic secondary NH group, and another singlet at δ 6.59 ppm shows existence of one proton at C4 of pyrazole ring. Apart from above signal rest of the signals were observed in the aromatic region. 13C signals of 4a recorded a singlet at δ 161.54 ppm due to and another signal at δ 171.2 ppm due to C8 carbon of triazolothiadiazole that reveals expected cyclization, while other signals of 13CNMR spectra of 4a were obtained at predicted chemical shifts values. Moreover the % of C, H, N and S were establish to be 56.40, 2.98, 17.74, 5.70 respectively, which also indicates that it is in good conformity with molecular formulae C26H16BrN7OS. Base peak in GC-MS (m/z) at [M+2] +at 556also supported the formation of target compound. The nonappearance of characteristic absorption peaks due to -NH2 and -SH groups in 4a as earlier shown in 3 clearly confirmed its formation. The entire synthesized heterocyclic compounds 4a-g was assessed for their in-vitro antimicrobial activity. Results obtained are summarised in the Table No. 2 and 3.
Table 2: Antibacterial screening of (4a-i)
|
Zone of Inhibition (mm) |
||||||||||||
|
|
Gram +ve | Gram –ve | ||||||||||
|
Compd. Code |
||||||||||||
|
Conc. (μg/mL) |
||||||||||||
| 1000 | 500 | 250 | 125 | 63.5 | 31 | 1000 | 500 | 250 | 125 | 63.5 |
31 |
|
|
4a |
24 | 21 | 19 | 18 | 17 | 14 | 26 | 23 | 20 | 18 | 16 | 12 |
| 4b | 22 | 20 | 19 | 18 | 16 | 14 | 24 | 22 | 21 | 19 | 17 |
15 |
|
4c |
20 | 19 | 17 | 15 | 14 | 12 | 25 | 21 | 19 | 16 | 15 | 12 |
| 4d | 21 | 18 | 16 | 15 | 13 | 11 | 22 | 20 | 18 | 15 | 14 |
13 |
|
4e |
25 | 21 | 19 | 17 | 16 | 15 | 23 | 22 | 21 | 19 | 16 | 14 |
| 4f | 24 | 21 | 20 | 19 | 18 | 16 | 27 | 25 | 22 | 18 | 15 |
13 |
| 4g | 22 | 20 | 19 | 17 | 16 | 14 | 23 | 21 | 20 | 17 | 15 | 14 |
| DMSO | – | – | – | – | – | – | – | – | – | – | – | – |
| Std. Drug | 24 | 22 | 20 | 19 | 17 | 15 | 28 | 24 | 20 | 17 | 16 | |
Table 3: Antibacterial screening of (4a-i)
| Zone of Inhibition (mm) | ||||||||||||
| Compd. Code | Gram -ve | |||||||||||
|
E. coli |
S. typhi |
|||||||||||
|
Conc. (μg/mL) |
||||||||||||
| 1000 | 500 | 250 | 125 | 63.5 | 31 | 1000 | 500 | 250 | 125 | 63.5 |
31 |
|
| 4a | 26 | 24 | 22 | 19 | 17 | 15 | 16 | 15 | 13 | 10 | 09 |
07 |
|
4b |
23 | 21 | 20 | 18 | 16 | 14 | 14 | 12 | 11 | 09 | 08 | 06 |
| 4c | 24 | 22 | 19 | 17 | 15 | 13 | 15 | 13 | 10 | 08 | 07 |
05 |
|
4d |
26 | 23 | 22 | 20 | 18 | 15 | 12 | 10 | 09 | 07 | 06 | 04 |
| 4e | 25 | 24 | 21 | 18 | 17 | 14 | 15 | 14 | 12 | 11 | 09 |
07 |
|
4f |
24 | 23 | 20 | 17 | 16 | 13 | 13 | 12 | 10 | 08 | 07 | 05 |
| 4g | 20 | 19 | 17 | 16 | 14 | 12 | 14 | 13 | 11 | 10 | 08 |
06 |
|
DMSO |
– | – | – | – | – | – | – | – | – | – | – | – |
| Std. Drug | 26 | 24 | 23 | 21 | 17 | 14 | 17 | 15 | 12 | 11 | 09 |
08 |
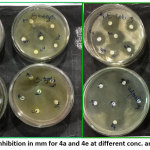 |
Figure 1: Zone of Inhibition in mm for 4a and 4e at different conc. and bacterial strains Click here to View Figure |
Conclusion
In conclusion, highly efficient and facile synthesis of novel series of 6-amino thiadiazole (4a-g) derivatives is described. The presented series of compounds were synthesized in excellent yields without any additional reagents or catalyst. The structure and purity of innovative compounds obtained was established by spectroscopic study and chemical assessment. Among the synthesized compounds maximum compounds exhibited reasonable to good activities on selected strains S. aureus, E. coli and P. vulgaris while poor activity was assessed for S. typhi.
Acknowledgements
The authors appreciate Dr. Roshan Nasare, Dr. Syed Abrar Ahmed and Dr. Mandar Paingankar, for their support and permission to carry out antimicrobial activities. Authors are also obliged to the Principal, Government Science College, Gadchiroli for providing laboratory conveniences. Authors are gratified to Director IIT Bombay and SAIF, Chandigarh for spectral analysis of compound.
Conflict of Interest
The authors announce that there is no conflict of interest.
References
- Tarik, E.; Mohammed, E.Eur. J. Chem.2010, 1, 6-11.
- Seelam, N.; Shrivastava, S.; Prasanthi, S.; Gupta, S.J. Saudi. Chem. Soc.2016, 20, 411-418.
- Singh, G.; Sharma, P.; Dadhwal, S.;Garg, P.; Sharma, S.Int. J. Curr. Pharm. Res. 2011, 3,105-118.
- Sheikhy, M.; Jalilian, A.; Novinrooz, A.; Motamedi-Sedeh, F.J. Biomed. Sci. Eng.2012, 5, 39-42.
- Singh, R.; Chouhan, A.World Pharma. Pharmace. Sci. 2014,3, 874-906.
- Kaushik, A.; Ganguly, S.; Sahu, J. K.J. Adv. Pharma. Tech. Res.2014, 5(2), 90-95.
- Hameed, A.; Hassan, F.Int. J. App. Sci. Tech. 2014, 4, 202-211.
- Menendez, C.; Gau, S.; Lherbet, C.; Rodriguez, F.; Inard, C.Eur. J. Med. Chem.2011, 46, 5524-5531.
- Bekircan, O.; Kucuk, M.; Kahveci, B.; Kolayli, S. Arch. Pharm. 2005,338, 365-372.
- Sztanke, K.; Tuzimski, T.; Rzymowska, J.; Pasternak, K.; Kandefer-Szerszeń, M. Eur. J. Med. Chem. 2008, 43, 404-419.
- Baviskar, B. A.; Khadabadi, S. S.; Deore, S. L.; Shiradkar, M. R. Der. Pharmacia. Sinica.2012, 3,24-30.
- Zitouni, G.; Kaplancikli, Z.A.; Erol, K.; Kilic, F.S. Farmaco, 1999, 54, 218-223.
- Zitouni, G.T.; Kaplancikli, Z.A.; Ozdemir, A.; Chevallet, P.; Kandilci, H.B.; Gumus, B. Arch. Pharm. 2007, 340, 586-590.
- Dong, W.; Liu, Z.; Liu, X.; Li, Z.; Zhao, W. Eur. J. Med.Chem.2010,45, 1919-1926.
- Farghaly, A.R.; El-Kashef, H. Arkivoc.2006, 11, 76-90.
- Zou, Y.; Zhao, Q.; Liao, J.; Hu, H.; Shichong, Y.; Chai, X.; Xu, M.; Qiuye, W. Bioorg. Med. Chem.2012,22, 2959-2962.
- Uchil, V. R.; Joshi, V.Ind. J. Chem. 2002, 41, 631-634.
- Shiv, K.; Gupta, P.K.; Sharma, M.; Bansal, Kumar, B.E.J. of Chem. 2011,8(2),594-597.
- Li, Q.; Ren, J.; Dong, F.; Feng, Y.; Gu, G.; Guo, Z.Carbohydr. Res.2013, 373, 103-107.
- Bai, J. K.; Zhao, W.; Li, H. M.; Tang,Y. J.J. Tang. Curr. Med. Chem.2012, 19, 927-936.
- Khan, I.; Ali, S.; Hameed, S.; Rama, N.H.; Hussain, M. T. Eur. J. Med. Chem.2010, 45, 5200-5207.
- Salgin-Goksen, U.; Gokhan-Kelekci, N.; Goktas, O.; Koysal, Y.; Kilic, E.Bioorg. Med. Chem.2007, 15, 5738-5751.
- Yusuf, M.;Khan, A. R.; Ahmed, B. Bioorg. Med. Chem. 2008, 16(17), 8029-8034.
- Sarafroz, M.; Khatoon, Y.; Ahmad, N.; Amir, M.; Pottoo, H. F.Orient. J. Chem.2019, 35(1), 64-70.
- Wujec, M.; Pitucha, M.; Dobosz, M.; Kosikowska, U.; Malm, A. Acta. Pharm.2004, 54,251-260.
- Sanmati, K.; Mishra, P.J.Computational Method Mol. Des.2011, 1(1),52-58.
- Karakus, S.; Coruh, U.; Barlas-Durgun, B.; Ezequiel, M.; Vazquez-Lopez, Ozbas-Turan, S.; Akbuga, J.; Rollas, S. Marmara. Pharm. J.2010, 14, 84-90.
- Joseph Raj, X.; Rjendran, N. Int. J. of Electrochemical Sci. 2011, 6, 348-366.
- Matysiak, J. Eur. J. Med. Chem. 2007, 42, 940-947.
- Bhusari, K. P.; Khedekar, P.B.; Umathe, S. N.;Bahekar, R. H.; Raghu Ram Rao, A. Ind. J.Hetero.Chem.2000, 9(4),275-278.
- Siddiqui, N.J.; Idrees M.; Khaty, N.T.; Dhonde, M.G.S., Am. J. Pharm. Tech. Res., 2015,5(1), 290-300.

This work is licensed under a Creative Commons Attribution 4.0 International License.









