New Series of Substituted Heterocyclics Derived from α , β – Unsaturated Ketone and Their Cytotoxic Activity Tumor Cell Lines
Muna S. AL-Rawi , Huda A. Hassan, Dheefaf F. Hassan and Ismaeel Y. Majeed
, Huda A. Hassan, Dheefaf F. Hassan and Ismaeel Y. Majeed
Department of Chemistry, College of Education For Pure Science Ibn Al- Haitham, University of Baghdad, Baghdad-Iraq.
Corresponding Author E-mail: tagreedaloom@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340620
Article Received on : 30-09-2018
Article Accepted on : 06-11-2018
Article Published : 10 Nov 2018
The aldol condensation of 2-acetylnaphthalene with 9-anthracene carboxaldehyde afforded α, β-unsaturated keton (1) . New heterocyclic compounds containing: cyclohexenone[2], indazole[3], pyrimidinethion [4], thiazolo fused pyrimidine[5], isoxazoline[6], substituted pyrazoline[7]a-d and pyrimidinone[8] rings system were synthesized from α, β-unsaturated keton[1]. Cyclization of [1] with ethylacetoacetate gave the mentioned heterocycle cyclohexanone [2]. The cyclo condensation of [2] with hydrazine gave the new indazole derivative [3]. furthermore, the reation of [1]with thiourea gives thiopyrmidine derivative [4]. The cyclo condensation of [4] with chloroacetic acid gave the fused rings [5]. Then reacted compound[1] with hydroxylamine to produce isoxazoline [6]. Also the reaction of [1]with hydrazine and hydrazide derivatives to produce pyrazole [7]a-d. All the newly synthesized derivatives[2-7a-d] were characterized via the CHN-S, FT- IR, 1H NMR, and13C NMR (of some of theme). The antibacterial and cytotoxic activities were evaluated for these derivatives[2-7a-d] . The study of antibacterial displayed good to moderate activity and the study of anticancer activity showed that were effective for inhibition of L20 B the mice intestines carcinoma cell line and RD human pelvic rhabdomyosarcoma cell line. Treated.
KEYWORDS:α, β-Unsaturated ketons; Anti-Cancer Activity; Cyclohexenone; Indazole; Isoxazoline; Pyrazoline; Pyrmidinone;Pyrimidinethion
Download this article as:| Copy the following to cite this article: AL-Rawi M. S, Hassan H. A, Hassan D. F, Majeed I. Y. New Series of Substituted Heterocyclics Derived from α,β – Unsaturated Ketone and Their Cytotoxic Activity Tumor Cell Lines. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: AL-Rawi M. S, Hassan H. A, Hassan D. F, Majeed I. Y. New Series of Substituted Heterocyclics Derived from α,β – Unsaturated Ketone and Their Cytotoxic Activity Tumor Cell Lines. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=52371 |
Introduction
α,β – Unsaturated ketons, one of the primary classes of natural products and belongs to flavonoid family, as well these compounds keep several biological activities.1,2 α,β -Unsaturated ketons are suitable for the synthesis of an important heterocycles rings like cyclohexenone, indazole, pyrimidinethion, isoxazoline, pyrazoline, and pyrimidinone.3 The biological activity for chalcones are wide ranging in the recent years, isoxazolines are considered biologically active molecules since they are used in controlling parasite infections in humans4 in addition to their use as insecticides,5 nematicides and molluscicides agents.6 Pyrazolines, for example, have attracted increasing attention due to their pharmaceutical applications such as: anti-bacterial, anti-fungal, enzymatic inhibitors and cytotoxic properties.7-9 Pyrmidine derivatives play an essential role in medicinal field due to their anti-inflammatory, anti-ulcerogenic.10 The anti-inflammatory and anti-cancer activities of indazoles were also reported (11). Hence, it appeared of interest to prepare the mentioned heterocycles linked to 2-acetyl naphthalene moiety and evaluated their cytotoxicity effect on two cancer cell lines including: L20 B and RD cell lines.
Experimental
Materials
All chemicals utilized brand Sigma-Aldrich and used as received.
Instrumentation
FTIR spectra were registered in KBR dices on a SHIMADZU-FTIR-8400spectro photometer. 1H& 13CNMR spectra were done on Bruker 400- MHz spector photometer. CHN-S were completed an EuroEA Elemental Analyzser.
General Synthetic Procedures
All compounds [2-8] were synthesized confer to the scheme 1.
![Scheme 1: Synthetic route for target derivatives [1-8].](http://www.orientjchem.org/wp-content/uploads/2018/11/Vol34No6_Ser_Mun_sch1-150x150.jpg) |
Scheme 1: Synthetic route for target derivatives [1-8]. |
Preparation of of chalcone: 3-(anthracen-9-yl)-1-(naphthalen-2 -yl) prop–2 –en -1- one [1]
2-Acetyl naphthalene (1.70g, 0.01 mol) was added with small portions to a solution of (2.06g, 0.01 mol) 9-anthracenecarboxaldehyde in 10% ethanolic NaOH (10 mL) and stirred for 3h. The reaction mixture was acidification with dilute HCl. The resulting product was filtered off, washed well with cold water, dried in air to give the required product [1]; Yield 82%; mp 155-157°C; FT- IR : 3049 (C-H arom.), 2931 ( C-H aliph.), 1658 (C=O), 1622- 1597 (C=C) and 1172due to( C-O-C); 1H- NMR (δ): 8.42-7.70 (m, 16H, Ar-H), 6.20, 6.15 (2H,d, CH=CH). Furthermore, the results of CHN-S analysis harmonized with the proposed structure for [1] C27H18O: C, 90.47; H, 5.06; Found:C, 90.91; H,5.63.
Synthesis of ethyl 6-(anthracen-9-yl)-4-(naphthalen-2-yl)-2-oxocyclohexa-3-ene-carboxylate [2]
A stirred mixture of chalcone [1] (3.58g, 0.01 mol), CH3COOC2H5 (0.01mol), aqueous KOH solution (1mL, 10%) was heated for 3h and then left to stirring overnight at ambient temperature. The precipitated off white was collected and dried to give the cyclohexanone derivatives [2]; yield : 68% ; m.p. 182-1840C; FTIR 3035 (C-H arom.), 2981 ( C-H aliph.), 1737 (C= O) 1660 (C =O) and 1604 (C =C); 1HNMR : 8.55-6.92 (m, 16H, H-Ar ), 6.69(s,1H, CH=C-Ar),4.02(1H,m,CH-Ar/), 2.71 ( 1H, s, CH-CO), 1.58 (s, 2H, CH2-C-Ar), 1.24 (q,2H, -CH2CH3) and 1.10 (t,3H, -CH2CH3); Anal.Calcd. for C33H26O3: C( 84.23); H(5.57); Found:C(84.85); H (5.76).
Synthesis of 4-(anthracen-9-yl)-6-(naphthalen-2- yl) -2 ,3 ,4 ,5 -tetrahydro-3H-indazol-3-one [3]
Hydrazine hydrate was added dropwise to a solution of compound[2] (4.70 g,0.01 mol) in ethanol (20 mL) and glacial acetic acid (0.3 mL) at ambient temperature. The mixture was heated for 6h(12). The pale yellow solid formed was filtered off and dried , yield:52%; m.p. 137-1390C; FT-IR(3385-NH), (3020C-H arom.), (2980 C-H aliph.), (1653 C = O) (1631 C = N) and (1955 C=C); 1HNMR (9.78:s,1H,NH),(7.86-7.22 : m, 16H, H-Ar ), (4.02:s, 1H, CH =C-Ar), (3.47 :1H, d, CH-CO), 3.18(d,2H, CH2CH-Ar/ ), 1.35 (m, 1H, CH2-CH- Ar/); likewise, the results of CHN-S analysis agree with the suggested structure for compound(3) :C31H22N2O: (C:84.91); (H:5.06); (N:6.39) ;Found:(C:85.15); (H:5.66,( (N:6.91).
Synthesis of 4-(anthracen-9-yl)-6-(naphthalen-2 -yl) – 3 , 4-dihydropyrimidine 2 (1H) -thione [4]
To a solution of chalcone (3.58g) in ethanolic NaOH (0.01 mol), SC(NH2)2 (0.6 g) was added, refluxed for 7 hr. A solution of ice cold water was added, the orange solid created, washed with water and dried, yield :68%; m.p. 214-216 0C ; FT-IR: 3383cm-1 for(NH), 3012cm-1 (C-H arom.), 1638 (C =N), and 1255 (C =S). 1HNMR: 12.00 (s,1H, NH tautomeric with SH in thiopyrimidine ring), 11.38(s,1H,NH),9.00- 7.86(m, 16H ArH), 8.29 (d ,1H, CH = C-Ar ), 7.65 (d ,1H, = CH-CH-Ar); Also, the output of CHN-S analysis correspond with the indicate structure for C28H20N2S.
Synthesis of 5-(Anthracen -9 -yl)-7-(naphthalen- 2- yl) -5H- Thiazolo [3,2-a] pyrimidin-3(2H)- one [5]
Chloroacetic acid (0.01mol) was added to a compound [4] (4.16g,0.01mol) in mixture of glacial CH3CO2H (10 ml) and (CH3CO)2O (5ml) and anhydrous sodium acetate (0.45 g). The mixture refluxed for 6h and poured into 30 mL crushed ice to allow golden yellow solid precipitate, which was filtered and dried; Yield (48%); m.p. 193-1950C; FT-IR( ν ,cm-1): 3091 (C-Haromatic), 2098 (C-H aliphatic), 1668 (C= O), 1606 (C= N), 1078(C-S); 1H NMR (9.55- 7.16 :m, 16H Ar-H), 8.46 (d, 1H, CH=CAr ), 5.45 (d,1H, =CH-CH-Ar), 1.66(s,2H,CH2). CHN-S for compound C30H20N2OS: (C, 78.92); (H, 4.42); (N, 6.14); (S, 7.02);Found: (C,79.20); (H,4.18); (N,6.45); (S,7.42).
Synthesis of derivatives 5-(anthracen-9-yl) – 3 – (naphthalene – 2 – y l ) -4 ,5-dihydro -isoxazole[6]
To a mixture of chalcone[1] (3.58g, 0.01mol) and hydroxyl amine hydrochloride (0.69g, 0.01mol) ethanolic sodium hydroxide (10mL) was refluxed for 8h. On cooling 50 mL water was added then the mixture acerbated at fridge overnight and the white solid was formed, yield ( 77% ); m.p. 146-1480C; FT-IR3064 (C-H arom.), 1627 (C=N), 1598 (C= C); 1348 (cyclic ether) (C – O); 1HNMR: (8.89 -7.36: m, 16H, ArH), (4.73-4.61 :d, 2H, CH2CHAr/), (2.25-1.20 :t,1H, CH2-CH- Ar/). The CHN analysis for C27H19NO: (C, 86.84); (H, 5.13); (N, 3.75); Found: (C,86.22); (H,5.45); (N,3.95).
Synthesis of 5-( naphthalen -2-yl)-3- (substituted phenyl) – pyrazoline derivatives [7] a-d
A mixture of chalcon [1] (3.58g, 0.01mol) and hydrazine hydrate or substituted hydrazine (0.01 mol) in EtOH (20 mL) containing glacial acetic acid (0.3 mL) was heated for 4h. The precipitated was formed, collected and dried to afford [7]a-d.
5-(anthracen-9-yl)-3-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazole[7]a
Yellowish brown; yield ( 58% ); m.p. 92-940C; FTIR for this compound confirm that:3431(NH), 3053 (CH -Ar), 1666 (C =N), 1593 (C = C); 1HNMR : (9.01:s,1H, NH), (8.48 -6.76: m, 16H Ar-H), 4.11 (t,1H, CH2CHAr/ ), 2.49-1.46 (d,2H, CH2-CH- Ar/), CHN analysis (C27H20N2) agree with the proposed structure: C,87.07; H,5.41; N,7.52 Founder: C,87.55; H,5.85; N,7.85.
5-(anthracen-9-yl) -3- (naphthalen-2 –yl ) -1- phenyl-4, 5- dihydro -1H-pyrazole[7]b
dark orange; yield (60%); m.p. 149-1510C; FTIR(CH-Ar 3057), (C =N1620); 1HNMR , 8.12 -7.23 (m, 16H ArH), 3.67 (t,1H, CH2CHAr/ ), 1.19-1.35 (d,2H, CH2-CH- Ar/); the CHN-S compound[7]b C33H24N: C,88.36; H,5.39; N,6.25, Founder: C,88.99; H,5.70; N,6.05.
(2-aminophenyl)(5-(anthracen-9-yl)-3-(naphthalen-2-yl) -4 , 5- dihydro-1H-pyrazol -1- yl) methanone [7]c
Brownish red; yield ( 74% ); m.p. 172-1740C; FTIR: 3344(NH2), 3026 (CH -Ar), 1666 (C =N), 1593 (C =C) , 1336 (C =S) ; 1HNMR : 9.32(s,1H,NH), 8.70 -7.55 (m, 16H, Ar-H), 4.27 (t,1H, CH2CHAr/ ), 1.711.21 (d,2H, CH2-CH- Ar/); CHN-S for compound C34H25N3O [7]c: C, 83.07; H, 5.13; N, 8.55; Found: C, 83.65; H, 5.42; N,8.76.
1-(5-(anthracen-9-yl)-3-(naphthalen-2-yl)-4,5-dihydro -1H– pyrazol -1 -yl) thiourea [7]d
bright yellow; yield ( 67% ); m.p. 204-2060C; FT-IR( ν ,cm-1): 3309(NH2), 3035 (C-H aromatic),1718(C=O), 1660 (C=N), 1597 (C =C); 1HNMR: 11.47(s,1H,NH2), 9.70 -7.45 (m, 16H Ar-H), 7.60-7.61 (t,1H, CH2CHAr/ ), 1.62 (d,2H, CH2-CH- Ar/);Anal. Calcd. for C28H22N4S: C, 75.31; H, 4.97; N, 12.55; S, 7.18 Founder: C, 75.73; H, 5.11; N,12.32;S,7.29.
Synthesis of 4-( anthracen -9 –yl )-6-( naphthalene -2 -yl)-3,4-dihydropyrimidin- 2(1H)-one [8]
To a solution of chalcone [1] (3.58g, 0.01 mol), urea (0.6g, 0.01 mol) and EtOH (30 mL) containing glacial CH3CO2H (0.3ml) was added.The combination was heated for 7h and poured into crushed ice. The solid light yellow precipitate was washed, filtered and dried to give pyrimidinone derivative[8]. Yield (75% ); m.p. 119-1210C ; FTIR: (3406,3350NH), (3064 CH arom.), (1670 C=O), ( 1614,1597 C=C); 1HNMR: 10.12 (s,1H, NH ),8.82(s,1H, NH), 8.38-7.35 (m,16H, Ar-H and pyrimidine proton ), 3.33 (d,1H, =CH-CH ); 13CNMR the presence of peaks at δ 190.88 (C=O), δ 125.11-138.96 (C aromatic) and δ 123 (CH=CH) ; CHNS for C28H20N2O the target compound: C, 83.98; H, 5.03; N, 7.00; Founder: C,83.67; H,5.43; N,7.11.
Results and Discussion
Aldole condensation reaction of 2-acetyl naphthalene with 9-anthracenecarboxaldehyde in alcoholic sodium hydroxide offorded3-(anthracen -9- yl)-1-(naphthalen-2-yl) prop-2-en-1- one [1] at room temperature. The structural of the chalcone [1]was substantiated spectroscopically and elemental analyses . The formation of cyclohexanone derivative [2] was achieved by refluxing chalcone[1] with ethylacetoacetate in the presence of aqueous potassium hydroxide solution. Moreover, reaction of this derivative[2] with hydrazine hydrate in the presence of CH3CO2H as acatalyst give a new indazol derivative[3],the reaction may have proceeded through condensation between carbonyl group of cyclohexanone[2] and amineNH2 of hydrazine, followed by cyclization by losing a molecule of ethanol. The pyrimidinethione derivative [4] was obtained from refluxing chalcone [1] with thiourea in basic medium, its structure was ascertained by spectral data. However, compound [5] was prepared directly from the reaction [4] with ClCH2CO2H in glacial CH3CO2Hand (CH3CO)2O in the presence of anhydrous CH3CO2Na. Isoxazoline compound [6] was synthesized from the reaction of chalcone [1] with NH2OH.HCl. Sutructure of compound [6] was established by their spectral data, the FTIR spectra were no characteristic bands of the CH=CH and C=O and NH groups in the starting material, with the appearance of new absorption band for (C=N) group around 1627 cm-1and C-O (cyclic ether) group around 1348 cmˉ1. It was reported that, reaction of α, β-Unsaturated ketone with N2H4 or PhNHNH2 or 2-amino benzohydrazide or thiosemicarbazide in the presence of glacial acetic acid and refluxed ,yield the target pyrazoline [7]a-d, respectively. The structure of the pyrazoline derivatives [7]a-d were identified by their melting point, CHN-S analysis, FTIR and 1HNMR spectroscopy. The FTIR spectra of these compounds exhibited new absorption stretching peaks of NH and imiene groups in the region 3431cm-1 and 1666-1660cm-1, the more characteristic for pyrazole ring. The pyrimidinone derivative [8] was synthesized from reaction of chalcone [1]with urea in glacial acetic acid as a catalyst. The structure of the pyrimidinone [8] characteristic by FTIR, H& 13C NMR spectroscopy; the suggested mechanism of this reaction may be shown as follows Scheme 2.
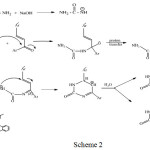 |
Scheme 2 |
Antibacterial Evaluation
The target compounds were synthesized [2-8]were screened their antibacterial activity (in vitro) against both G+ and G- bacteria; like Staph. aureus(G+), Bascillus cereus (G+) and E.coli(G-) according to the agar plate diffusion method. The test solution was prepared by using DMSO as a solvent, and each compounds was dissolved in DMSO to give concentration (1ppm). The plates were then incubated at 37°C and examined after 24 hrs. (+) and (+ +) depending upon the diameter and clarity as in Table 1. The derivatives [3],[5],[7]b,[7]d, and [8] displayed good to moderate antibacterial activity against three pathogenic species; also, the derivatives [2],[6],[7]a and [7]c shows low activity against Gram positive. Finally, both compounds[2] and[7]c shows no activity against Gram negative but they exhibited moderate to weak antibacterial against Gram poistive.
Table 1: antibacterial activity of the synthesized compounds[2-8].
|
.Comp No. |
Staphlococcus aurus(G+) |
Bascillus cereus (G+) |
E. Coli (G‐) |
.Comp No. |
Staphlococcus aurus(G+) |
Bascillus cereus (G+) |
E. Coli (G‐) |
| [2] | 7.1 | 12 | 0 | [7]a | 5.5 | 15 | 6.9 |
| [3] | 20.4 | 16.8 | 6.5 | [7]b | 27 | 7.7 | 9.8 |
| [4] | 11.7 | 6.6 | 13.7 | [7]c | 10 | 7.5 | 0 |
| [5] | 18.7 | 13 | 11.9 | [7]d | 19.2 | 11 | 8.8 |
| [6] | 8.1 | 9.5 | 8 | [8] | 13.7 | 11.8 | 6.7 |
Key to symbols: highly active = ≤15 mm, moderately active =11-15 mm and slightly active =5-10mm.
Cytotoxic Activity
All derivatives of chalcone were chosen for examined their anticancer activity. Two cell lines were used for testing: L20B (mice intestines carcinoma) cell line and RD (human pelvic rhabdomyosarcoma) according to the procedure of Freshney.13 Seven derivatives [2-8] different concentrations were tested for their cytotoxicity by using cultured cells in microtiter plate (96wells). The examine was applied by the next steps:
a-Seeding
When cells in the incubated falcon became monolayer, a liquot 200 µl/104-105 cells/well from single cell hang were added to all the 96 wells of the microtiter plates; which wrapped by plate lids and sealed with parafilm, shaked softly and returned to the incubator.
b- Incubation
Plates were incubated in moisten chamber at 37°C, 5% CO2.
c- Exposure
When the cells are in full activity, they were exposed to 6 concentrations of the derivatives µg/ml for cell line, 200µl of maintenance medium were added to each well of control group, then plates were locked with parrafilm and returned to the incubator. cytotoxicity was carried out after 48 hr and the photo picture were taken after 24 hr.
d- Staining
Cell viability was measured after 48 hr, then removing the medium and 20 µl/well solution of MTT were added. The crystals remaining in the wells were solubilized by the addition of 200 µl/well of DMSO followed by incubation in 37°C for 15 min with shaking. The absorbance was measured on a microplate reader at 620 nm. The average of inhibition of cell growth was counted according to (46) as follow equation:-
![]()
the synthesized derivatives [5],[7]a,[7]b,[7]c and [8]showed moderate anticancer activity against two type of cancer cell lines. The remaining derivatives exhibited low anticancer activity. However, the compound [6] showed no activity versus both cell lines, and [2] showed no activity versus RD cell line. Structure-activity relationship could be observed by examining the effect of heterocyclic rings on these derivatives on the cell growth inhibition.
Table 2: The inhibition of cells growth of synthesized derivatives. µl/well.
| Comp. No. | for (L20 B) | for (RD) | Comp. No. | for (L20 B) | for (RD) |
| [2] | 20.0% | – | [7]a | 47.8% | 42.1% |
| [3] | 15.9% | 23.3% | [7]b | 60.7% | 33.5% |
| [4] | 11.5% | 25.7% | [7]c | 18% | 11.9% |
| [5] | 52.7% | 12.2% | [7]d | 51.9% | 32.3% |
| [6] | – | – | [8] | 23% | 34.2% |
Conclusions
The aim of the present work has been directed towards synthesized of new series of heterocyclic compounds derived from α, β-un saturated ketone they may be have more activity and less toxicity as anticancer agents. Biological evaluation may support the development of synthetic organic compoundes and pharmaceutical chemistry. The study of antibacterial displayed good to moderate activity. All compounds except [6] showed moderate to low inhibition for L20B and RD cancer cell lines.
Acknowledgment
We thank Dr. Hytham Ahmed -Faculty of Pharmacy -Menoufia University/ Egypt for support us throughout our experimental work.
References
- N. Beyhan, B.Kocyigit-Kaymakcioglu, S. Gu, and F. Aricioglu, Arabian Journal of Chemistry, (2017) 10, S2073–S2081.
CrossRef - N.Prakash, M.Elamaran and N. Ingarsal, Chemical Science Transactions, (2015), 4(4), ISSN: 2278-3458,947-954.
- . Muna S. AL-Rawi, Huda A. Hassan, Dheefaf F. Hassan and Rana M. Abdullah, International Journal for Sciences and Technology,( 2013),Vol. 8, No.2, 48.
- Sanjay Menon, Susan M. K. Shaheen and Valerie A.Lain Court, “Spiro -cyclic Isoxazolines As Anti-parasitic Agents”, WO Patent, 039484 A1, (2012).
- P hillip G.Lahm , Wagerle T. and Ming X.,”Isoxazoline Insecticides ” , US Patent, 0127165 A1, (2014).
- David J. Calderwood, Eric C. Breinlinger and Steven L. Swann, Subhendu Makherjee, ” Isoxazolines As Therapeutic Agents”, WO Patent, 151158A1, (2012).
- Oren M. Becker , Shtrit A., Schutz N., Zeev B., Yacovan A. and Ozeri R.,” Pyrazolines for the Modulation of PKM2″, US patent , 0302609A1,(2013)
- Patrich Page, Francesca Gaggini and Benoit laleu, “Pyrazoline Dione Derivatives As NADPH Oxidase Inhibitors “, US Patent, 0172352 A1, 2012
- Mohamed EL. K., Malika B., Bouabdallah I., Abdelmajid Z.,Fouad M., Rachid T., Abdelkrim R. and AHMED M.; Natural Product Research, Vol. 21, No. 11, 2007, 947.
CrossRef - Casey C. Maccomas , Scott D. Kuduk and Thomas S. Reger,”Pyrmidine PDE10 Inhibitors”, WO Patent , 081619A1, (2014).
- Zacharia Cheruvallath, Philip Erckson and Jun feng, ” Indazole derivatives “, WO Patent, 130855A1,(2013).
- Nehad A. Abdel Latif, Manal M. Saeed , Nesreen S. Ahmed,Rasha Z. Batran and Nadia R. A. El-Mouhty; International Journal of Innovative Research in Science,Engineering and Technology; (2014),Vol. 3, Issue 1,8517.
- Freshney RI., “Culture of Animal Cells: A manual of Basic Technique and Specialized Applications,” 6th Edition, Wiley: New York, 2010.

This work is licensed under a Creative Commons Attribution 4.0 International License.









