Green Synthesis of antibacterial Zinc oxide Nanoparticles using biopolymer Azadirachtaindica gum
A.Geetha1, R. Sakthivel2, J. Mallika1*, R. Kannusamy 2and R. Rajendran3
1Department of Chemistry,PSG College of Arts and Science, Coimbatore-641014,India
2Department of Electronics,PSG College of Arts and Science, Coimbatore-641014,India
3Department of Microbiology,PSG College of Arts and Science, Coimbatore-641014,India
Corresponding author E-mail: jmpsgche@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320222
Article Received on :
Article Accepted on :
Article Published : 11 Apr 2016
The progress of green chemistry in the synthesis of nanoparticles with use of plants has engrossed a great attention nowadays due to its inexpensive, simple, non-toxic and environmental-friendly nature. The present study has been undertaken to prepare Azadirachtaindicagum stabilized ZnO nanoparticles with multifunctional properties. The prepared nanoparticles were characterized by FT-IR, XRD, FE-SEM and UV-Vis absorption studies. It was clear from XRD pattern that nanoparticles were crystallized in hexagonal wurtzite structure. The average size of the nanoparticles was found to be 30-60nm. The synthesized nanoparticles exhibited potent antibacterial activity against E. coli and S. aureus.
KEYWORDS:Zinc oxide nanoparticles; Azadirachtaindica(AI); Green synthesis; Antibacterial activity
Download this article as:| Copy the following to cite this article: Geetha A, Sakthivel R, Mallika J, Kannusamy R, Rajendran R. Green Synthesis of antibacterial Zinc oxide Nanoparticles using biopolymer Azadirachtaindica gum. Orient J Chem 2016;32(2). |
| Copy the following to cite this URL: Geetha A, Sakthivel R, Mallika J, Kannusamy R, Rajendran R. Green Synthesis of antibacterial Zinc oxide Nanoparticles using biopolymer Azadirachtaindica gum. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15251 |
Introduction
Nanotechnology is emerging as a rapidly growing field with its application in science and technology for the purpose of manufacturing new material at the nanoscale level.1Nanotechnology involves synthesis, characterization and application of nanoparticles by controlling shape and size. In the field of nanotechnology, metal nanoparticles have attained a great importance due to their unique optical, catalytic, electronic, magnetic and antimicrobial properties owing to their small size.2-4 Among the metal oxide nanoparticles, zinc oxide is interesting because of its physical and chemical properties such as stability, high catalytic activity, luminous transmittance, effective antibacterial property, intensive IR and UV absorption etc. 5,6 Interestingly, ZnO nanoparticles are reported by several studies as non-toxic to human cells.7,8 This aspect necessitatedtheir usage as antibacterial agents, noxious to microorganisms and hold good biocompatibility to human cells.9 Synthesis of nanoparticles can be performed using a number of routinely used chemical methods such as chemical precipitation10, sonochemical, solovothermal, sol-gel process, hydrothermal decomposition etc.11The biological method of the synthesis of ZnO nanoparticles is gaining importance due to its simplicity, eco-friendliness and extensive antimicrobial activity.12
The use of plant materials has been considered as a green route for the biosynthesis of nanoparticles owing to their environmental friendly nature. Plant based gum is a heteropolysaccharide of natural gum and its morphological, thermal, compositional, physico-chemical properties have been studied well.13-15 The biopolymer of Azadirachtaindicagum has the highest amount of proteins found amongst all plant gums.16 Mukherjee and Srivastava reported that, the major constituents ofneem gum were found to be polysaccharides such as D-galactose-glucuronic acid, D-xylose, L-arabinose and L-fucose. The naturally occurring Azadirachtaindica gum exudates is a easily available, non-toxic, glassy transparent brown colour, water soluble complex polysaccharide which has various applications in paper, cosmetic, textile and pharmaceutical industries.
The presence of acetyl, carboxyl, hydroxyl and carbonyl functional groups and metal- biosorption properties of the neem gum encouraged us to use this AI gum exudates as a biotemplate/stabilizer for the green synthesis of ZnO nanoparticles. The present investigation describes the advantages of ZnO nanoparticles synthesized by the green biological method using AI gum, their characterization and the determination of antibacterial activity.
Materials and Methods
Chemicals
All the chemical such as zinc nitrate hexahydrate (ZnNO3.6H2O) and sodium hydroxide (NaOH) was of analytical grade and used without further purification. All the synthesis was carried out using double distilled water.
Synthesis of zinc oxide nanoparticles
Chemical method
The bulk zinc oxide particles were prepared by using wet chemical method. In this method zinc nitrate (0.1 M) was prepared in distilled water under constant stirring for an hour using magnetic stirrer. Stirring was continued for another 2 hours with the drop by drop addition of 0.2 M sodium hydroxide solution. The content was allowed to settle for overnight and the supernatant was then discarded carefully. The impurities were removed by washing and centrifuging several times with distilled water. The pure nanoparticles were dried at 80◦C for overnight in a hot air oven. The complete conversion of zinc hydroxide into zinc oxide takes place during drying.
Green method
Collection and purification of Azadirachtaindica gum
Dried gum exudates of Azadirachtaindica were collected from Neem trees in the campus of PSG College of Arts and Science (Coimbatore, India). The collected gum was dissolved using double distilled water, filtered and kept in dessicator to get a glassy mass of purified gum.
The zinc oxide nanoparticles were prepared by green method using zinc nitrate and sodium hydroxide as precursors and AI gum as stabilizing agent. Different concentrations of AI gum 0.1%, 0.3% and 0.5% were prepared in 100 ml. To this solution 2.97 g of zinc nitrate was added to get 0.1 M solution. The contents were kept under constant stirring using magnetic stirrer to dissolve the zinc nitrate. Sodium hydroxide (0.2 M) was added drop wise and stirring was continued for 2 hours. The solution was allowed to settle for overnight, centrifuged and the supernatant was discarded. Thus obtained nanoparticles were washed several times with distilled water to remove the impurities. The nanoparticles were dried at 80◦C for overnight in hot air oven where the complete conversion of zinc hydroxide into zinc oxide takes place.
Characterization techniques
UV-Visible absorption spectroscopy is widely being used technique to examine the optical properties of nanosized particles and band gap is the major factor determining the electrical conductivity. The UV-Vis spectrums of ZnO nanoparticles synthesized by chemical and green method were recorded using JASCO Corp., V-570 spectrophotometer over a range of 200-800 nm. The presence and interaction of chemical functional groups were analyzed using FT-IR spectrophotometer (Perkin Elmer) at the scanning range of 4000-400 cm-1. The X-ray powder diffraction pattern were recorded on an X-Ray diffractometer (XRD, PW 3040/60 Philips) with Cu (kα) radiation (λ=1.5406 A◦) operating at 40 kV and 30 mA with 2θranging from 20o– 90o. The surface morphology of ZnO nanoparticles were characterized by FE-SEM using Carl Zeiss Supra 55 (Germany) microscope.
Antibacterial activity of ZnO nanoparticles
The agar well diffusion method was used to screen the antibacterial activity of bulk ZnO and AI gum stabilized ZnO nanoparticles. For this purpose, Gram-negative bacteria: Escherichia coli and Gram-positive bacteria: Staphylococcus aureus were employed in the present study.
Muller Hinton agar was prepared and sterilized. Log phase culture of the test specimens were swabbed over the agar surface using the sterile cotton swab. Wells were made on the agar surface using sterile gel puncture and about 10µl of the sample were loaded onto the wells and the plates were incubated at 37o C for 24 hours. The clear zones were used to determine the efficiency of the samples.
Results and Discussion
UV-Vis absorption studies
The UV-Vis absorption spectra of ZnO nanoparticles obtained by green method are compared with ZnO nanoparticles prepared using chemical method. The absorption spectra of bulk ZnO nanoparticles and AI gum stabilized ZnO nanoparticles were shown in Fig.1.The absorption wavelength and band gap energy of bulk ZnO nanoparticles and different concentrations of AI gum stabilized ZnO nanoparticles were shown in Table .1. Analysis of the data shows the absorption wavelength obtained for ZnO prepared by chemical method is higher than greener method. ZnO nanoparticles prepared using greener method with various concentration of AI gum shift the surface Plasmon resonance band to lower wavelength side (blue shift). This blue shift in the AI gum stabilized ZnO nanoparticles is due to the presence of complex organic molecules carrying different charge centers in AI gum.
Table 1: Absorption wavelength and band gap energy of bulk ZnO and AI gum stabilized ZnO nanoparticles
| Particulars of ZnO |
Wavelength ( nm) |
Band Gap energy (eV) |
|
Bulk ZnO |
372 |
3.33 |
|
0.1 % AI gum stabilized ZnO |
362 |
3.43 |
|
0.3 % AI gum stabilized ZnO |
359 |
3.46 |
|
0.5 % AI gum stabilized ZnO |
355 |
3.49 |
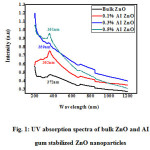 |
Figure 1: UV absorption spectra of bulk ZnO and AI gum stabilized ZnO nanoparticles |
The absorption wavelength and the size of the different concentrations of AI gum stabilized ZnO nanoparticles decreases as the concentration of AI gum increases. The concentration of AI gum increases, band gap energy also increases.
FT-IR Spectra
The role of varying AI gum concentration in the formation of ZnO nanoparticles were identified by comparing the FT-IR spectrum of AI gum (Figure.2), and FT-IR spectra of bulk ZnO and ZnO nanoparticles synthesized using various concentrations of AI gum as stabilizing agent (Figure.3).
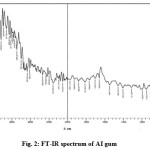 |
Figure 2: FT-IR spectrum of AI gum Click here to View figure |
The peak in between 3600-3200 cm-1 in the FT-IR spectrum of AI gum (Figure.2) indicates the presence of higher amount of hydroxyl groups in the polysaccharides of gum. The observed peak at 1705 cm-1 indicates the >C=O stretching frequency. The peaks at 1658 cm-1 and 1550 cm-1represents the carbonyl stretching vibration and N-H stretching vibration of amine groups in peptides.
The peaks at 1458 cm-1 and 1311 cm-1 show the presence of C-H bond. A medium stretch at 1080 cm-1represents the presence of C-O bond. From the FT-IR spectra, for bulk ZnO (Figure.3), the broad band between 3200-3600 cm-1 centered at 3402 cm-1 corresponds to the stretching vibration of intermolecular hydrogen bond (O-H) existing between the adsorbed water molecule and oxygen of zinc oxide. The peak at 1627 cm-1 corresponds to O-H bending vibration. The peaks at 424 cm-1 indicates the stretching vibration of Zn-O bond.
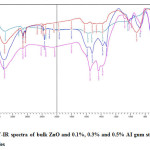 |
Figure 3: FT-IR spectra of bulk ZnO and 0.1%, 0.3% and 0.5% AI gum stabilized ZnO nanoparticles Click here to View figure |
The FT-IR spectra of ZnO nanoparticles synthesized using 0.1%, 0.3% and 0.5% AI gum as stabilizing agent is given in Fig.3. A broad band between 3600-3200 cm-1 centered at 3402 cm-1in ZnO nanoparticles were shifted to 3425, 3448 cm-1 in the spectrum of ZnO nanoparticles synthesized using AI gum as stabilizing agent. The higher shift frequency from 3402 cm-1 to 3425, 3448 cm-1 may be due to the participation of –OH group from the bulk molecule of AI gum. The peaks at 2924 and 2854 cm-1 are due to C-H stretching vibration. The N-H stretching vibration of AI gum appeared at 1658 cm-1 is lowered to 1589, 1581 and 1550 cm-1 in the spectra of ZnO nanoparticles synthesized using AI gum.17-19 Thus there is a possibility of interaction between the proteins of AI gum and ZnO nanoparticles.
The stretching of vibration of C-O at 1085 cm-1 in AI gum is lowered to 1018,1026 and 1041 cm-1 in the ZnO nanoparticles synthesized using AI gum as stabilizing agent indicating the formation of new bond. The peak appeared for bulk ZnO nanoparticles at 424 cm-1 is shifted to higher frequency side in the presence of AI gum stabilized ZnO nanoparticles. The shift depends on the concentration of AI gum used. The values are 563, 524, 501 and 439 cm-1 for 0.1%, 0.3% and 0.5% AI gum stabilized ZnO respectively. The higher frequency shift may be an indication of structural changes in the ZnO nanoparticles in presence of AI gum.
X-ray diffraction studies
X-Ray diffraction is a well known technique for the structural identification and determination of crystalline size.
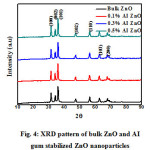 |
Figure 4: XRD pattern of bulk ZnO and AI gum stabilized ZnO nanoparticles |
The XRD pattern of synthesized bulk ZnOand AI gumstabilized ZnO were shown in Figure.6. The prominent peaks corresponding to the diffraction planes (100), (002), (101), (102), (110), (103) and (200) were obtained. The comparison of observed XRD pattern with the standard JCPDS card data indicates that, all the peaks are matched with standard JCPDS card no.36-1451, confirms that the bulk ZnO and AI gum stabilized ZnO nanoparticlesare of hexagonal wurzite type structure.20, 21 Crystallite size (D) was calculated using Debye Scherrer, s formula22
D= 0.9λ/β, where D- crystalline size of zinc oxide, λ – wave length of X- ray source 0.15406 nm in (XRD), β – full width at half maximum of the diffraction peak, θ- Bragg angle
The average crystallite size (D) of the prepared ZnO nanoparticles was calculated to be around 13 – 15 nm.
FE-SEM studies
FESEM images of ZnO nanoparticles synthesized by chemical method were shown in Figure. 5a, 5b. Analysis of the images shows that formation of zinc oxide nanoparticles ranging from 50-90 nm with flower shape morphology.
 |
Figure 5a, 5b: FE-SEM images of ZnO nanoparticles Click here to View figure |
ZnO nanoparticles synthesized using AI gum as stabilizing agent was shown in Figure. 6a – 6f.
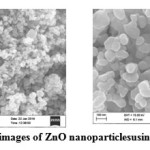 |
Figure 6a, 6b: FE-SEM images of ZnO nanoparticles using0.1% AI gum Click here to View figure |
 |
Figure 6c, 6d: FE-SEM images of ZnO nanoparticlesusing 0.3% AI gum Click here to View figure |
 |
Figure 6e, 6f: FE-SEM images of ZnO nanoparticles using 0.5% AI gum Click here to View figure |
The images show the same with the average particle size of 30-60 nm and with spherical shape and this also matched with the earlier studies.3, 23 Thus, the nanoparticles using AI gum as stabilizing agent decreased the particle size.
Antibacterial studies
The nanoparticles of ZnO exhibit attractive antibacterial propertiesdue to increased specific surface area as the reduced particle size leading to enhanced particle surface reactivity and contact with the microbial pathogens. The smaller size of nanoparticles facilitates easy entry into the microbial cell membrane and enables inhibition mechanisms to occur inside the cell. ZnO nanoparticles generate hydrogen peroxides which chemically interact with membrane proteins and lipid bilayers.24-25 The ZnO nanoparticles may distort and damage the bacterial cell membrane, causing leakage of intracellular contents leading to cell death.
The zone ofinhibition for both Gram-negative bacteria Escherichia coli and the Gram-positive bacteria Staphylococcus aureus were calculated by measuring the diameter of the inhibited growth around the wells. The antibacterial activities of ZnO nanoparticles against the studied pathogenic strains are shown in Fig. 7. The values of zone of inhibition obtained from the assay are presented in Table.2.It is quite interesting to note that all bacterial species tested in this study showed resistance to ZnO nanoparticles synthesized by green method than the bulk ZnO. Both Gram-negative and positive bacteria had shown good sensitivity and it is most significant for the 0.5% AI gum green synthesized ZnO nanoparticles for the concentration 10 μg/mL.
Table 2: Antibacterial activity of bulk ZnO and 0.1%, 0.3% and 0.5% AI gum stabilized ZnO nanoparticles
|
Testorganisms |
Zone of inhibition in mm |
|||
|
ZnO |
0.1% AI-ZnO |
0.3% AI- ZnO |
0.5% AI- ZnO |
|
| E. coli |
5 |
5 |
8 |
10 |
| S. aureus |
5 |
8 |
10 |
10 |
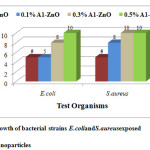 |
Figure 7: Growth of bacterial strains E.coli and S.aureus exposed Click here to View figure |
Conclusion
The present study demonstrates ZnO nanoparticles have been successfully synthesized using different concentrations of AI gum as stabilizing agent. UV-Vis, FT-IR, XRD and FE-SEM analysis were used to confirm the formation of ZnO nanoparticles by chemical and green method. The synthesized ZnO nanoparticles has an average particle size of about 30-60 nm and particles are nearly uniform with hexagonal wurtzite structure and this conforms the ability of the AI gum to stabilize the ZnO nanoparticles. The synthesized ZnO nanoparticles showed potent antibacterial activity against Gram-negative bacteria Escherichia coli and Gram-positive bacteria Staphylococcus aureus. These results concluded that the green synthesized ZnO nanoparticles using Azadirachtaindica gum extract could be used as a potent antibacterial agent in various biomedical applications.
References
- Albrecht, M.A.; Evan, C.W.;Raston, C.L. Green chemistry and the health implications of nanoparticles, Green Chem, 2006, 8, 417–432.
CrossRef - Garima, S.; Bhavesh, R.; Kasariya, K.; Ranjan, A.S.; Singh, R.P. Biosynthesis of silver nanoparticles using Ocimum sanctum(Tulsi) leaf extract and screening itsantimicrobial activity, J Nanopart Res, 2011,13, 2981–2988.
CrossRef - Vidhya, C.; Shilpa, H.; Chandraprabha, M.N.;Antonyraj, M.A.L.; Indu, V.G.; Aayushi, J.;Bansal, K. Green synthesis of Zinc Oxide Nanoparticles by Calotropisgigantea, IJCET, 2013, 118-120.
- Duran, N.; Marcato, P.D.; Alves, O.L.; Souza, G. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusariumoxysporum strains, Journal of Nanotechnology 2005, 3, 1–7.
- Ingle, A.; Gade, A.; Pierrat, S.; Sonnichsen, C.; Rai, M. Mycosynthesis of silver nanoparticles using the fungus Fusariumacuminatum and its activity against some human pathogenic bacteria,Current Nanoscience, 2008, 4, 141–144. Anand, K.; Siby Varghese and Thomas Kurian. Synthesis of znonanorods through mehcano-chemical route: A solvent free approach,Inter. J of Theo. and App. Sci,2014, 6(2), 87-93.
- Colon, G.;Ward, B.C.; Webster, T.J. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2,J. Biomed. Mater. Res, 2006,78(3), 595–604.
CrossRef - Avnishkumararora.;Saritadevi.;Viveksheeljaswal.; Jogindersingh.;Mayankkinger and Vishnu devgupta. Synthesis and Characterization of ZnO Nanoparticles, Orient j chem,2014, 30(4), 1671-1679.
- Padmavathy, N.; Vijayaraghavan, R.; Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study, Sci. Technol. Adv.Mater,2008, 9(3), 035004.
CrossRef - Hamid rezaghorbani.;Ferdosparsamehr.; Hosseinpazoki and Behradmosavarrahmani. Synthesis of ZnO Nanoparticles by Precipitation Method, Orient j chem, 2015, 31(2), 1219-1221.
- Kolekar, T.V., Bandgar, S.S., Shirguppikar, S.S., Ganachari, V.S. Synthesis andcharacterization of ZnO nanoparticles for efficient gas sensors, Arch. Appl. Sci. Res.2013, 5(6), 20–28.
- Gunalan, S., Sivaraj, R., Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens, Prog.Nat. Sci. Mater. Int,2012, 22 (6), 693–700.
CrossRef - Srivastava, V.K., Rai, R.S., Physico-chemical studies on gum Dhawa (Anogeissuslatifolia), Colloid Polym. Sci, 1963, 190, 140–143.
- Aspinall, G.O., Bhavanadan, V.P., Christensen, T.B., 1965. Gum ghatti (Indian gum)Part V. Degradation of the periodate-oxidised gum, J. Chem. Soc,1965, 2677–2684.
CrossRef - PalaniyandiVelusamy.; Jayabrata Das.; Raman Pachaippan.; Baskaralingam, Vaseeharan and KannaiyanPandian. Industrial Crops and Products,2015, 66, 103-109.
- Ogunjimi, A.T., Alebiowu, G., Flow and consolidation properties of neem gum co-processed with two pharmaceutical excipients, Powder Technol, 2013, 246, 187–192.
CrossRef - BusiSiddhardha, HnamteSairengpuii and RajkumariJobina, Biogenic synthesis of silver nanoparticles using aqueous floral extract of Azadirachtaindica and its Anti-candida and larvicidal activities, Res. J. of Chemistry and Environment, 2015, 19(6), 20-27.
- KasiMurugan, BalakrishnanSenthilkumar, DuraisamySenbagam, SalehAl-Sohaibani, Biosynthesis of Silver nanoparticles using Acacia leucophloea extract and their antibacterial activity, International Journal of Nanomedicine, 2014, 9, 2431-2438, 2014.
- Sashi, P.D, Manu, L., Mika, S. Green synthesis and characterization of silver and gold nonoparticles using leaf extract of Rosa rugosa, Colloids Surf., A, 2010, 364, 34-41.
CrossRef - Zhou, J. Zhao, F.; Wang, Y.; Zhang, Y.; Yang, L. Plectranthusamboinicusleaf extract mediated synthesis of zinc oxide nanoparticlesand its control of methicillin resistant Staphylococcus aureus biofilm and bloodsucking mosquito larvae, J. Lumin.,2007, 123, 195-202.
CrossRef - Khoshhesab, Z.M.; Sarfaraz, M.; Asadabad, M.A. Investigation of phosphonicacidsurface modifications on zinc oxide nanoparticles under ambient conditions, Syn. React. Inorg. Met.,2011, 41, 814-820.
CrossRef - Cullity, B.D. Elements of X-Ray Diffraction, Addison-Wesley, Reading, Mass, USA, 3rd edition, 1967.
- Anand raj, L.F.A andRajalakshmy, E. Biosynthesis and characterization of zinc oxide nanoparticles using root extract of Zingiberofficinale, Orient j chem, 2015,31, 51–56.
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, AVR. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids surf.,B, Biointerfaces,2012, 94, 143-150.
CrossRef - Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.M.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. International journal of nanomedicine,2012,7, 845-857.

This work is licensed under a Creative Commons Attribution 4.0 International License.









