Towards the First Total Synthesis and Anticancer Screening of Polycarponin C: A Cyclic Octapeptide
Nirmala V. Shinde*1 ,Avinash S. Dhake2 and Kishan P. Haval3
1Department of Pharmaceutical Sciences, Jawaharlal Nehru Technological University, Kakinada, Andhra Pradesh, India
2Department of Pharmaceutical Chemistry, S.M.B.T. College of Pharmacy, Nandi Hills, Dhamangaon. Tah: Igatpuri, Dist: Nashik (MH) India.
3Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Subcentre Osmanabad(MH) India.
Corresponding Author Email: nirmalampharm@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/320159
Article Received on :
Article Accepted on :
Article Published : 09 Feb 2016
The present study describes designing, synthesis and anticancer screening of a proline rich cyclic octapeptide polycarponin C, by solution phase synthesis. The synthesis was carried out by coupling a tetrapeptide Boc-Pro-Thr-Leu-Pro-OH with another tetrapeptide Boc-Pro-Val-Leu-Phe-OH, followed by cyclization of the linear fragment.The structure of the synthesized compound was then confirmed by spectral analysis. From the results of biological activity, it was concluded that, the compound had shown moderate activity against SR human tumor cell lines of Leukemia when compared with Vincristine as a standard.
KEYWORDS:cyclic peptide; solution phase synthesis; p-nitro phenyl ester method; coupling agent; anticancer
Download this article as:| Copy the following to cite this article: Shinde N. V, Dhake A. S, Haval K. P. Towards the First Total Synthesis and Anticancer Screening of Polycarponin C: A Cyclic Octapeptide. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Shinde N. V, Dhake A. S, Haval K. P. Towards the First Total Synthesis and Anticancer Screening of Polycarponin C: A Cyclic Octapeptide. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=13931 |
Introduction
Anticancer drug development from natural sources has always been fascinating and interesting for researchers working in the field of medicinal chemistry and drug development. Owing to the various biological activities like antimicrobial, cytotoxic, anti-HIV, anti-inflammatory, serine protease and protein tyrosine phosphatase inhibitory activity possessed by cyclic peptides1-7 and to obtain a natural bioactive peptide in good yield, in the present work an attempt has been made towords the first total synthesis of a proline rich cyclic octapeptide polycarponin C, cyclo-( Pro-Thr-Leu-Pro-Pro-Val-Leu-Phe), isolated from whole plants ofPolycarpon prostratum, belonging to family Caryophyllaceae8. The synthesis was attempted by solution phase technique9 followed by cyclization of linear fragment by p-nitro phenyl ester method12. The structure of the synthesized compound was confirmed by detailed spectral analysis. The synthesized compound was then subjected for preliminarey cytotoxic activity by using Brine shrimp assay, and further followed by screening against 60 human tumor cell lines at NCI, USA. The results of anticancer screening showed that the compound is moderately active against SR human tumor cell lines of Leukemia when compared with Vincristine as a standard.
Materials and Methods
All L-amino acids, di-tertbutyldicarbonate (Boc2O),diisopropylcarbodiimide (DIPC), trifluoroacetic acid (TFA), triethylamine (TEA), pyridine and N-methylmorpholine (NMM) were purchased from Spectrochem Limited (Mumbai, India).Melting points were determined by using digital melting point apparatus.
The IR spectra were run on FTIR spectrophotometer,JASCO 4100 using a thin film supported on KBr pellets or utilizing chloroform and NaCl cells.1H NMR and 13C NMR spectra were recorded on Bruker AC NMR spectrometer using DMSOas a solvent. The MASS spectrum of the cyclopeptide was recorded on JMS-DX 303 Mass spectrometer operating at 70 eV by ESIMS/MS.
In order to carry out the total synthesis of cyclicpeptide, polycarponin C, cyclo-(Pro-Thr-Leu-Pro-Pro-Val-Leu-Phe), it was disconnected into four dipeptide units, Boc-Pro-ThrOMe 1, Boc-Leu-Pro-OMe 2, Boc-Pro-Val-OMe 3, Boc-Leu-Phe-OMe 4. The dipeptides were obtained by coupling Boc amino acids with the respective amino acid methyl esters, by using DIPC as a coupling agent. The ester group of dipeptide 1 and 3 was then removed by using LiOH and the Boc group of dipeptide 2 and 4 was removed by using TFA. The deprotected units were then coupled to get two tetra peptides Boc-Pro-Thr-Leu-Pro-OMe 5 and Boc-Pro-Val-Leu-Phe-OMe 6. The resulting tetrapeptide were then coupled together by using DIPC and chloroform to obtain a linear octapeptide, which was then cyclized by using p-nitro phenyl ester method to get titled compound.
General method for preparation of Di/Tetra/linear octapeptide peptide
L-Amino acid methyl ester hydrochloride/dipeptide methyl ester/tetra peptide methyl ester (10 mmol) was dissolved in chloroform (CCl3, 20 ml). To this, TEA (2.8 ml, 20 mmol) was added at 0 °C and the reaction mixture was stirred for 15 min. Boc-L-amino acid/Bocdipeptide/ Boctetrapeptide (10 mmol) in chloroform (20 ml) and DIPC (10 mmol) were added with stirring. After 24 h, the reaction mixture was filtered and the residue was washed with chloroform (30ml) and added to the filtrate. The filtrate was washed with 5% NaHCO3 and saturated NaCl solutions.The organic layer was dried over anhydrous Na2SO4, filtered and evaporated in vacuum. The crude product was recrystallized from a mixture of chloroform and petroleum ether (b.p. 40-60 °C) followed by cooling at 0 °C.
By using above procedure, compound 1-7 were synthesized.
Procedure for cyclization of linear octapeptide12
The cyclization of linear octapeptide was attempted by using p-nitro phenyl ester method. The ester group of linear fragment was removed with LiOH and the p-nitro phenyl ester group was introduced. For introduction of p-nitro phenyl ester group, the Boc-peptide carboxylic acid(1.5 mmol) was dissolved in chloroform (15 ml) at 0 °C, to which p-nitro phenol(0.27 gm, 2 mmol) was added, and stirred for 12 hrs at RT. The reaction mixture was filtered and the filtrate was washed with NaHCO3 solution (10%) until excess of p-nitro phenol was removed and finally washed with 5% HCl (5 ml) to get Boc-peptide-pnp ester.
To the above Boc–peptide–pnp–ester (1.2 mmol) in CHCl3 (15ml), CF3COOH (0.274g, 2.4 mmol) was added, stirred for 1 hourat room temperature and washed with 10% NaHCO3 solution. The organic layer wasdried over anhydrous Na2SO4.To the Boc–deprotected peptide–pnp–ester in CHCl3 (15ml), N-methyl morpholine (1.4ml, 2mmol.) was added and kept at 00C for 7 days. The reaction mixture was washed with 10% NaHCO3 until the byproduct p–nitrophenol was removed completely and finally washed with 5% HCl (5ml). The organiclayer was dried over anhydrous Na2SO4.Chloroform and pyridine were distilled off to get the crude product of the cyclized compound, which was recrystallized from CHCl3/n–hexane.
Anticancer activity of synthesized compound:
Preliminary cytotoxic activity by Brine Shrimp Lethality Assay (BSLA)10, 11
Brine shrimp eggs were obtained from the aquarium shop, Nasik. Artificial sea water was prepared from (1% NaCl) nitrate, phosphate, and silicate-free sea-salt and distilled water (35 g/l) at 25°Cunder constant illumination. The saltwater solution was aerated continuously during incubation with an aquarium air pump. The seawater was put in a small plastic container (hatching chamber) with a partition for dark (covered) and light areas. Shrimp eggs were added into the dark side of the chamber while the lamp above the other side (light) will attract the hatched shrimp. Two days were allowed for the shrimp to hatch and mature as nauplii (larva). After two days, when the shrimp larvae are ready, 4 ml of the artificial seawater was added to each test tube containing different conc. of drug and 10 brine shrimps were introduced into each tube. Thus, there were a total of 30 shrimps per dilution. Then the volume was adjusted with artificial seawater up to 5 ml per test tube. The test tubes were left uncovered under the lamp. The number of surviving shrimps were counted and recorded after 24 hours. The lethality concentration (LC50) was assessed at 95% confidence intervals. The percentage mortality (%M) was also calculated by dividing the number of dead nauplii by the total number, and then multiplied by 100%. This is to ensure that the death (mortality) of the nauplii is attributed to the activity of the compound. The results of activity are shown in Table 1.
In vitro cytotoxic activity against Human tumor cell lines12:
The synthesized compound was screened for in vitro anticancer assay by National Cancer Institute (NCI), Bethesda, USA in a panel of 60 humantumor cell lines. The screening of the compound operated with In Vitro Cell Line Screening Project (IVCLSP), which is dedicated service, providing direct support to the DTP anticancer drug discovery program. The screening was carried out against 60 different human tumor cell lines of the leukemia, Non-small cell lung, colon, CNS, melanoma, ovarian, renal, prostrate and breast cancers which was aimed in showing selective growth inhibition or cell killing of particular tumor cell lines by specific compound. The screening begins with the evaluation of selected compounds at a single dose of 10-5 M.The output from the single dose screen is reported as a mean graph and is available for analysis by the COMPARE program.
The result of anticancer screening is shown in fig. 1 and Table 2.
Results and Discussion
Spectral characterization
Physical state: Semisolid mass
IR Data:intense N-H and C=O abortions at 3300 and 1650 cm-1respectively
3427, 1650 cm-1 are due to amino and amide carbonyl groups respectively
FABMS showed M+ ion peak at m/z 860, which matches to the molecular formula C46H68O8N8
13C NMR showed six amide carbonyl carbon
δ 171 for C=O of Thr, δ 62.5 for Cα ofThr, δ 66.5 for Cβ ofThr, δ 22 for Cγ of Thr, δ 172 for C=O of Phe,δ 52.9 for Cα ofPhe, δ38 for Cβ ofPhe, δ 171.5 for C=O of Val, δ 62 for Cα of Val, δ 31.5 for Cβ of Val, δ 19 for Cγ of Val, δ 170.5 for C=O of Leu, δ 50 for Cα of Leu, δ 41.5 for Cβ of Leu, δ 25.1 for Cγ of Leu, δ 172.5 for C=O of Leu2, δ 51 for Cα of Leu2, δ 43.5 for Cβ of Leu2, δ 25for Cγ of Leu2, δ 173 for C=O of Pro1, δ 62.2 forCα of Pro1, δ 32.2 forCβ of Pro1, δ 22.2 for Cγ of Pro1, δ 171 for C=O of Pro2, δ 61.2 for Cα of Pro2, δ 31 for Cβ of Pro2, δ 22for Cγ of Pro2, δ 170.8 for C=O of Pro3, δ 59.2 for Cα of Pro3, δ 28.6 for Cβ of Pro3, δ 25.8 for Cγ of Pro3
1H NMR: showed five amide N-H signals
δ 7.2 for HN of Thr, δ 4.55 for Hα of Thr, δ 4.65 for Hβ of Thr, δ 1.36 for Hβ of Thr, δ 11.04 for HN of Phe, δ 5.02 for Hα of Phe, δ 3.01 for Hβ of Phe, δ 8.7 for HN of Val, δ 4.7 for Hα of Val, δ 2.5 for Hβ of Val, δ 1.5 for Hβ of Val, δ 8for HN of Leu1, δ 5.36 for Hα of Leu1, δ 1.74 for Hβ of Leu1, δ 1.90 for Hβ of Leu1, δ 8.5 for HN of Leu2, δ 5.05 for Hα of Leu2, δ 1.7 for Hβ of Leu2, δ 1.94 for Hβ of Leu2, δ 4.45 for Hα of Pro1, δ 2.16 for Hβ of Pro1, δ 1.8 for Hβ of Pro1, δ 4.4 for Hα of Pro2, δ 1.90 for Hβ of Pro2, δ 1.65 for Hβ of Pro2,
δ4.39 for Hα of Pro3, δ 1.90 for Hβ of Pro3, δ 2.16 for Hβ of Pro3,, 4.08 for Hδ of Pro3.
Elemental analysis: C=63.96, H=8.4, N=13.10, O=14.91
Results of Biological activity
Table 1: Results for Cytotoxic activity by using Brine Shrimp Assay:
| Compound | Conc.(ppm or μg/ml) |
Number of SurvivingNapulii After 24 h |
Total Number ofSurvivors |
% Mortality |
||
|
T1 |
T2 |
T3 |
||||
| Poly C |
1000 |
4 |
6 |
4 |
14 |
53.33 |
|
500 |
5 |
5 |
5 |
15 |
50 |
|
|
250 |
5 |
6 |
7 |
18 |
40 |
|
|
125 |
7 |
6 |
7 |
20 |
33.33 |
|
|
62.5 |
8 |
9 |
8 |
25 |
16.66 |
|
|
31.25 |
10 |
10 |
10 |
30 |
0 |
|
*T=Trial LC50=460.9 μg/ml
Table 2: % GI shown by Polycarponin Cagainst different human tumor cell lines
|
Human Tumor Cell Line |
% GIfor comp. VIII
|
Human Tumor Cell Line |
% GIfor comp. VIII
|
| LeukemiaCCRF-CEMHL-60(TB)K-562RPMI-8226
SR |
15.3322.9939.1715.09
61.87 |
Ovarian CancerIGROV1 | 13.6 |
| Colon cancerHCT-116HCT-15HT29KM12
SW-620 |
12.3911.797.0113.9
8.69 |
Renal CancerUO-31 | 15.89 |
| CNS CancerSNB-75 | 18.20 | Breast CancerMCF7 | 31.78 |
| MelanomaLOX IMVIMALME-3MDA-MB-435 | 5.5912.8926.76 | MeanDelta Range | 90.5561.8798.09 |
*The compound had shown very poor activity against remaining cell lines.
 |
Figure 1: DTP One dose Mean graph for Polycarponin C Click here to View figure |
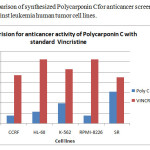 |
Figure 2: Comparison of synthesized Polycarponin Cfor anticancer screening with data of vincristine against leukemia human tumor cell lines. Click here to View figure |
 |
Scheme 1: Synthetic route for Polycarponin C Click here to View scheme |
Conclusion
The compound was synthesized with good yield by using solution phase technique and found show moderate activity against SR human tumor cell lines of Leukemia when compared with Vincristine as a standard. In consultation with literature survey, synthesis of analogs of this molecule may lead to the development of potent anticancer agent.
Acknowledgement
Authors are thankful to CSIR, Lucknow-CDRI, New Delhi for financial assistance. We also extend our thanks to S.M.B.T. College of Pharmacy, Dhamangaon, Nashik for providing necessary facilities to do the research work and SAIF, Chandigarh for spectral analysis.
References
- Shinde,N.V.;Himaja, M.;Bhosale, S.K.;Ramana, M.V.;Sakarkar, D.M. Indian J Pharm Sci.2008, 70(6), 695-852
CrossRef - Dahiya R.;Gautam H. Bulletin Pharm. Res.2011,1(1), 1-10
- Chaudhary S.; Kumar H.;Verma H.;Rajpoot A. Int J. PharmTech Res.2012,4(1), 194-200
- Kawagishi H.; Somoto A.;Kuranari J.; Kimura A.; Chiba S. Tetrahedron1993,34(21), 3439-3440
CrossRef - Chaudhary S.;Kumar H.;Verma H.;Rajpoot A. Int Journal of PharmTech Res.2012, 4(1), 194-200
- Hernández D. Eur. J. Org. Chem.2008, 3389–3396
CrossRef - Shinde N.V.;Himaja M.;Bhosale S.K.;Ramana M.V.;Sakarkar D.M. Asian J Chem.2010,22(2), 996-1000
- Ding, Z. T.; Zhou, J.; Tan, N.; Cheng, Y.; Deng, S. ActabotanicaSinica.2001, 43(5), 541-44
- Bodanszky M. and Bodanszky A., Practice of peptide synthesis;Springer-verlog Publishers. New York, (1984)
CrossRef - Bussmann R.;Malca G.; Glenn A. J. Ethnopharmacology.2011,137, 121-140
CrossRef - Houghton P.; Fang R.;Techatanawat I. Science Direct.2007,42, 377–387. www.nci.gov.in

This work is licensed under a Creative Commons Attribution 4.0 International License.









