NaBH4/(NH4)2SO4: A Convenient System for Reduction of Carbonyl Compounds to their Corresponding Alcohols in wet-THF
Saber Akbarzadeh, Davood Setamdideh* and Mohammad Alizadeh Hedayati
Department of Chemistry, Faculty of Sciences, Ahar Branch, Islamic Azad University, Ahar, Iran
DOI : http://dx.doi.org/10.13005/ojc/300461
Article Received on :
Article Accepted on :
Article Published : 16 Dec 2014
NaBH4 (1-2 equivalents) in the presence of (NH4)2SO4 (1-2 equivalents) reduces varieties of organic carbonyl compounds such as aldehydes, ketones and α,β-unsaturated carbonyl compounds to their corresponding alcohols. Reduction reactions were carried out in THF-H2O in high to excellent yields of products.
KEYWORDS:NaBH4; Reduction; Carbonyl compounds; (NH4)2SO4
Download this article as:| Copy the following to cite this article: Akbarzadeh S, Setamdideh D, Hedayati M. A. NaBH4/(NH4)2SO4: A Convenient System for Reduction of Carbonyl Compounds to their Corresponding Alcohols in wet-THF. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Akbarzadeh S, Setamdideh D, Hedayati M. A. NaBH4/(NH4)2SO4: A Convenient System for Reduction of Carbonyl Compounds to their Corresponding Alcohols in wet-THF. Available from: http://www.orientjchem.org/?p=5911 |
Introduction
Preparation of alcohols from the reduction of carbonyl functional groups are importance in organic chemistry 1 and a large number of methods including catalytic reduction 2, hydride homogeneous catalysis 3, heterogeneous catalysissystems 4 and transfer reagents 5 have been reported to be effective towards carbonyl compounds reduction. But, most chemists employ lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4). Controlling the reducing power of NaBH4 has been achieved by different types of modifications 6-13. A literature review also reveals that the reduction of carbonyl compounds can be achieved by NaBH4 in the presence of mineral protic solids or under wet conditions with some limitations. In continuing our efforts for the development of new reducing systems, 14 we decided to investigate the reducing properties of NaBH4 in the presence of (NH4)2SO4 as the co-reagent for the reduction of a variety of carbonyl compounds. We now report the results of a parallel investigation of the role of (NH4)2SO4 in the reduction of carbonyl compounds to their corresponding alcohols. Former, (NH4)2C2O4 has been proposed as a co-reactant.
Results and Discussion
benzaldehyde as a model compound has been reduced with one molar equivalent of NaBH4 in THF-H2O (3:0.3 mL) within 20 in the presence of 1 molar equivalent of (NH4)2SO4 at room temperature as shown in scheme 1.
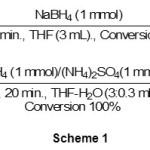 |
Scheme 1 Click here to View scheme |
The efficiency of this protocol was examined by the reduction of a variety of aldehydes. All reductions were completed within 20-80 minutes as shown in table 1, entries 1-7. The molar ratio of NaBH4 is not different according to the nature of the substrates. 1 molar equivalents of NaBH4 and 1 molar equivalents of (NH4)2SO4 per one equivalents of the substrate were sufficient to complete conversion of aldehydes to the corresponding alcohols in excellent yields (91-96%).
Our next attempt was the reduction of ketones to the corresponding secondary alcohols with the NaBH4/(NH4)2SO4 system. We optimized the reaction conditions with the reduction of the model compound acetophenone by NaBH4/(NH4)2SO4 under different conditions. Reduction of ketones was obtained with this reducing system, but due to the lower reactivity of ketones relative to aldehydes, the reduction require higher molar amounts of NaBH4 & (NH4)2SO4. The reduction reactions were performed with 2 molar amounts of NaBH4 in presence of 2 molar amounts of (NH4)2SO4 at room temperature in wet-THF as shown in scheme 2.
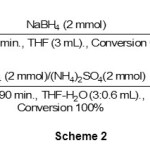 |
Scheme 2 Click here to View scheme |
All reductions were completed within 90-120 minutes with high to excellent yields (94-97%) as shown in table 1, entries 8-14.
The mechanism for the influence of (NH4)2SO4 is not clear, but we observed that with the addition of (NH4)2SO4 hydrogen gas slowly is liberated in situ. Hydrogen gas generation seems to be directly related to the solubility of the NaBH4 and (NH4)2SO4 in the reaction solvent. Because water causes an increase in solubility of the NaBH4 & (NH4)2SO4, so hydrogen gas generation increase dramatically.
Experimental
General
All substrates and reagents were purchased from commercially sources with the best quality. IR and 1H NMR spectra were recorded on PerkinElmer FT-IR RXI and 300 MHz Bruker spectrometers, respectively. The products were characterized by their 1H NMR or IR spectra and comparison with authentic samples (melting or boiling points). Organic layers were dried over anhydrous sodium sulfate. All yields referred to isolated pure products. The purity of products was determinate by TLC and 1H NMR. Also, reactions are monitoring over silica gel 60 F254 aluminum sheet.
A Typical Procedure for Reduction of Aldehydes with the Nabh4/(NH4)2SO4 System in Wet-THF
In a round-bottomed flask (10 mL) equipped with a magnetic stirrer, a solution of benzaldehyde (0.106 g, 1 mmol) in THF-H2O (3:0.3 mL) was prepared. To this solution, NaBH4 (0.038 g, 1 mmol) and then (NH4)2SO4 (0.132 g, 1 mmol) was added and the mixture was stirred at room temperature for 20 minutes. Completion of the reaction was monitored by TLC (Hexane/EtOAc: 9/1). Then, water (5 mL) was added to the reaction mixture and it was stirred for an additional 1 minute. The mixture was extracted with CH2Cl2 (3×10 mL) and dried over anhydrous Na2SO4. Evaporation of the solvent and a short column chromatography of the resulting crude material over silica gel (Hexane/EtOAc: 9/1) afforded the pure liquid benzyl alcohol (0.104 g, 96%, Table 1, entry 1).
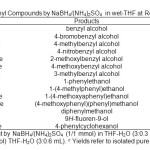 |
Table1: The Reduction of Carbonyl Compounds by NaBH4/(NH4)2SO4 in wet-THF at Room Temperature. Click here to View table |
A Typical Procedure for Reduction of Ketones with the Nabh4/(NH4)2SO4 System in Wet-THF.
In a round-bottomed flask (10 mL) equipped with a magnetic stirrer and a condenser, a solution of acetophenone (0.12 g, l mmol) in THF-H2O (3:0.6 mL) was prepared and NaBH4 (0.038 g, 1 mmol) and (NH4)2SO4 (0.234 g, 2 mmol) were added and the mixture was stirred at room temperature for 90 minutes. TLC monitored the progress of the reaction (Hexane/EtOAc: 9/1). After completion of the reaction distilled water (5 mL) was added to the reaction mixture and it was stirred for an additional 1 minute. The mixture was extracted with CH2Cl2 (3×10 mL) and dried over anhydrous sodium sulfate. Evaporation of the solvent and short column chromatography of the resulting crude material over silica gel (Hexane/EtOAc: 9/1) afforded the pure crystals of 1-phenylethanol (0.l1 g, 96% yield, Table 1, entry 8).
Conclusion
In conclusion, we have shown that the combination system of NaBH4/((NH4)2SO4 in wet-THF reduces a variety of carbonyl compounds to their corresponding alcohols in high to excellent yields at room temperature. Reduction reactions were carried out with 1-2 molar equivalents of NaBH4 in the presence of 1-2 molar equivalents of (NH4)2SO4. All reductions were accomplished with high efficiency of the reductions, using the appropriate molar ratios of NaBH4 and (NH4)2SO4, shorter reaction times and easy work-up procedure. Therefore this new protocol for reduction of carbonyl compounds could be a useful addition to the present methodologies.
Acknowledgments
The authors gratefully appreciated the financial support of this work by the research council of Islamic Azad University branch of Ahar.
References
- Ji, H. B. and She, Y. B. Green Oxidation and reduction, China Petrochemical Press, Beijing, 2005.
- Larock, R. C. Comprehensive Organic Transformations: a guide to functional group preparations, 2nd Ed.; Wiley-VCH, New York, 1999.
- Brandt, P.; Roth, P.; Anderssson, P. G., J. Org. Chem. 2004, 69, 4885-4890.
- Liu , P. N.; Gu, P. M.; Wang, F. D.; Tu, Y. Q., Org. Lett. 2004, 6, 169-172.
- Mohanazadeh F.; Hosini, M.; Tajbakhsh, M., Monatshefe fur Chemie. 2005, 136, 2041-2043.
- Hudlicky, M. Reductions in Organic Chemistry, Ellis Horwood Ltd. Chichester, 1984. (b) Seyden-Penne, J. Reductions by the Alumino and Borohydrides in Organic Synthesis, 2nd Ed.; Wiley-VCH, New York, 1997.
- ( a) Narayana, C.; Periasamy, M. Tetrahedron Lett. 1985, 26, 1757-1760.
- (a) Ranu, B. C. Synlett. 1993, 885-892. (b) Narasimhan. S; Balakumar, A. Aldrichimica Acta. 1998, 31, 19-26. (c) Soai, K.; Ookawa, A. J. Org. Chem. 1986, 51, 4000-4005.
- Firouzabadi, H.; Afsharifar, G. R. Bull. Chem. Soc. Jpn. 1995, 68, 2595-2602.
- Blough, B. E.; Carroll, F. I. Tetrahedron Lett. 1993, 34, 7239-7242.
- Zeynizadeh, B.; Setamdideh, D. Asian J. Chem. 2009, 21, 3603-3610.
- Zeynizadeh, B.; Setamdideh, D. Asian J. Chem. 2009, 21, 3588-3602.
- Zeynizadeh, B.; Setamdideh, D J. Chin. Chem. Soc. 2005, 52, 1179-1184..
- (a) Zeynizadeh, B.; Setamdideh, D Zeit. Natur. 2006, 61b, 1275-1281. (b) Zeynizadeh, B.; Setamdideh, D Synth. Commun. 2006, 36, 2699-2704.
- Setamdideh. D.; Ghahremani, S. S. Afr. J. Chem. 2012, 65, 91-97.

This work is licensed under a Creative Commons Attribution 4.0 International License.









