U.V., E.P.R. & X.R.D. Spectral Analysis of Bromo Mn(II) tetra seleniazide complex
Govind Kumar Gupta and S. P. S. Jadon*
Department of Chemistry, S.V. College, Aligarh (U.P.) 202001, India
DOI : http://dx.doi.org/10.13005/ojc/300221
Article Received on :
Article Accepted on :
Article Published : 04 Jun 2014
The complex of Mn (II) compound with Se4N3Br synthesized, was analyzed by U.V., E.P.R. and X.R.D. spectra. From the results, it is evident that the complex is good conductor, having paramagnetic character and orthorhombic geometry.
KEYWORDS:Se4N3Br; Orthorhombic; Geometry; Magnetic susceptibility
Download this article as:| Copy the following to cite this article: Gupta G. K, Jadon S. P. S. U.V., E.P.R. & X.R.D. Spectral Analysis of Bromo Mn(II) tetra seleniazide complex . Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Gupta G. K, Jadon S. P. S. U.V., E.P.R. & X.R.D. Spectral Analysis of Bromo Mn(II) tetra seleniazide complex. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3578" |
INTRODUCTION
The complexes of halogenated derivative of Se4N4 have been reported 1,2 . The adducts of Se4N3Cl with urea, thiourea, hydrazine and phenyl hydrazine have also been synthesized and reported 3,4. The Mass and I.R. spectral studies Mn (II), Fe(III), Co (II) complex with Se4N3Br have been reported by us 5-7. The present investigations are in continuation of previous work.
EXPERIMENTAL
To synthesis the complex, first of all Se4N3Br was prepared by the reaction of dry HBr on Se4N48,9 which was prepared by the ammoniation of SeBr4 in benzene. Equimoler ratio of MnCl2 and Se4N3Br (1:1) was mixed and refluxed for 6 hrs. in DMF. The grayish product formed, was separated, washed with D.M.F. and alcohol, dried and stored in vacuum desiccators over fused CaCl2.
U.V.,E.P.R, and X.R.D. spectra of complex, were recorded subsequently on Perkin-Elmer-Lambda-15 spectrophotometer (200-800nm) Varian’s X-E-4 band spectrometer at room temperature and PW-1710 X-ray power diffractometer usingλ =1.5418 , as source of radiation in the 2Θ range 0o-80 .
RESULTS AND DISCUSSION
On the basis of the quantitative ,Mass & I.R. spectral analysis the complex bromo Mn(II) tetra seleniazide has been assigned as (Se4N3Br)2MnCl2 4H2O (loc. site). The U.V. spectrum (fig-1) consist three prominent peaks at 200. 222.5, 282.5 nm. Former two bands 200, 222.5, corresponding 6.2, and 5.57ev energy, showing the ionic environment are due to the charge transfer transition in the complex. The last band, 282.5nm is on account of P∏ -d∏ transition for Se4N3 ring present in the complex. This is also confirmed from the frequencies ratios v1/ v2 =1.27 less than 2. The oscillator strength ‘f’ of the order 10-5(Table-1, Column-3) suggest the low spin Laporte forbidden transition in the complexes which is due to the sharing of electrons in Se4N3 ring. The low value of band gape energy,Δ Eg (Table-1,Column-4) and high value of number of conducting electrons, Nc of the order of 105 expounds the good conductive nature of complex.
 |
Fig1: U.V. spectrum of the Complex |
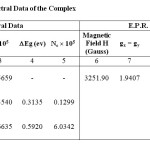 |
Table1: U.V. and E.P.R. Spectral Data of the Complex Click here to View table |
A single broad peak of high intensity has occurred in E.P.R. spectrum (fig-2) suggesting the paramagnetic character of complexes which is supported by the low value of magnetic susceptibility, =1.2352 10-3 e.s.u. (Table-1, Column-10). The value of gx = gy = 1.9407 (Table-1,Column-7) less than two explore the presence of vacant 3d energy shells in Mn atom to accept the electron pairs form N atom of Se4N3 ring, forming coordinate bond. The value of gz =2.0790 (Table-1,Column-8) greater than two indicate the sharing of electrons i.e. covalent bonding in Se4N3 ring of the complex.
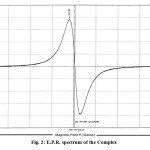 |
Fig2: E.P.R. spectrum of the Complex
|
Thus bromo Mn(II) tetra seleniazide, (Se4N3Br)2MnCl2 4H2O possess ionic bond due to Mn2+ and 2Cl–,coordinate bond, Se N Mn and Se N, Se Br covalent linkage with the 3d5 configuration of Mn2+ because the value of μeff=1.7215 is according to the presence of one unpaired electron. Thus the complex formed having quadridentated, coordinated linkage with square planar structure as reported (loc. site).
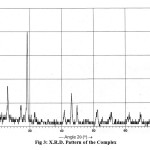 |
Fig3: X.R.D. Pattern of the Complex |
The X.R.D. pattern of complex recorded in 2θ range 0o-80 (fig-3) possesses a strong and intense peak at 29.21 for the Se4N3 ring. From the X-ray pattern the values of sin2 θ , miller indices, hkl, and inter planar distances ‘d’, calculated, resembles to the theoretical values (Table-2). From these X.R.D. data, the values of axial ratio and axial angles (Table-2) calculated are corresponding a0≠ b0≠ c0≠ and a = β = Y = 900 for the orthorhombohedral geometrical packing of the molecule in the complex.
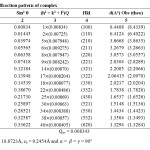 |
Table2: X-ray Diffraction pattern of complex Click here to View table |
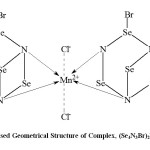 |
Fig4: Proposed Geometrical Structure of Complex, (Se4N3Br)2MnCl2.4H2O |
ACKNOWLEDGMENT
Authors wish to express to thank to the Director SAIF, Punjab University Chandigarh, Director SAIF, IIT Bombay, Director ACMS IIT Kanpur to provide instrumental facilities.
REFERENCES
- Thewalt, U.; Holl, K. Z. Naturforsch, 1984,Sec. B, 39, 145.
- Chivers, T. The Chemistry of Inorganic Ring Systems; Steudel,R. (Ed.), Elsevier Science Publishers, Amstardam, 1992, p. 409 .
- Dixit ,H.; Jadon, S.P.S. Int. J. Chem. Sci.,2005, 3(4), 709-714 .
- Dixit,H.; Jadon, S.P.S. Asian J. Chem.,2006, 18(1), 295 .
- Gupta, G.K.; Jadon, S.P.S. Int.J.Chem.Sci. 2009, 7(4), 2861-2866.
- Gupta, G.K.; Jadon, S.P.S. Int.J.Chem.Sci. 2012,10(2), 1091-1095.
- Gupta, G.K.; Jadon, S.P.S. Int.J.Chem.Sci. 2013,11(1), 306-312.
- Gowik,P. ; Klopotke, T. Spectrochim Acta A. 1990,46, 1371.
- Siivari, J.; Chivers,T.; Laitinen, R.S. Inorg. Chem.1993, 32, 1519.

This work is licensed under a Creative Commons Attribution 4.0 International License.









