Synthesis of N-Citralbenzenaminesbynabh4/B(Oh)3System
Davood Setamdideh* and Mohsen Minaee
Department of Chemistry, College of Sciences, Mahabad Branch, Islamic Azad University, Mahabad, Iran.
DOI : http://dx.doi.org/10.13005/ojc/300149
Article Received on :
Article Accepted on :
Article Published : 03 Mar 2014
Citral as α, β-unsaturated carbonyl compound has been reacted with structurally different anilines with sodium borohydride in the presence of boric acid for the synthesis of their corresponding N-citralbenzenamineswith good yields (75-85%)within 5 min in CH3CNat room temperature.
KEYWORDS:NaBH4; B(OH)3; Citral; N-citralbenzenamines
Download this article as:| Copy the following to cite this article: Setamdideh D and Minaee M. Synthesis of N-Citralbenzenaminesbynabh4/B(Oh)3System. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Setamdideh D and minaee M. Synthesis of N-Citralbenzenaminesbynabh4/B(Oh)3System. Orient J Chem 2014;30(1). Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2255 |
Introduction
Reduction of α, β-unsaturated carbonyl compounds can follow two pathways: addition to carbonyl group (1,2-reduction) to give allylicproducts or addition to the conjugated double bond (1,4-reduction) to give saturated carbonyl compounds. NaBH4 (common reducing agent) uses for the 1,2-reduction of conjugated carbonyl compounds under different reducing system 1-7.On the other hands, in the synthetic project we needed some N-citralbenzenamines.Also, recently we reported a convenient system for direct reductive amination of aldehydes by NaBH4/B(OH)3 system8. Therefore we decide to use of this reducing system for the synthesis of N-citralbenzenamines with this hope,the reductive amination of citralas anα, β-unsaturated carbonyl compoundfollow addition to carbonyl group (1, 2-addition) to give the corresponding α, β-unsaturatedN-benzenamines.
Results and Discussions
We have done the reductive amination reactions based on the optimized reaction that has been reported in the literature 7. These reactions were carried out with molar ratio of citral (1 mmol), anilines (1 mmol), B(OH)3 (1 mmol) and NaBH4 (1 mmol) in CH3CN (3 mL) at room temperature. The reactionswere completed within 5 min with 75-85% yields of product as shown in scheme 1. In this reactions N-(3,7-dimethylocta-2,6-dienyl)benzenamines(N-citralbenzenamines) as major products have been produced more than 75%;butgeraniol and unreacted anlinesas by-products less than 15%.
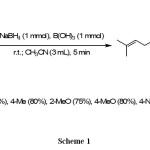 |
Scheme 1 |
Experimental
The products were characterized by their 1H NMR (400 MHz Bruker) or IR (PerkinElmer FT-IR RXI) and comparison with authentic samples (melting or boiling points). TLC was applied for the purity determination of substrates, products and reaction monitoring over silica gel 60 F254 aluminum sheet.
Reductive amination of citral and aniline with NaBH4/Al(OH)3, A typical procedure:
In a round-bottomed flask (10 mL) equipped with a magnetic stirrer, a solution of citral (0.152 g, 1 mmol), aniline (0.093 g, 1 mmol) and Al(OH)3 (0.078, 1 mmol) in CH3CN (3 mL) was prepared. The resulting mixture was stirred for 5 min at room temperature. Then the NaBH4 (0.036 g, 1 mmol) was added to the reaction mixture and stirred at room temperature. TLC monitored the progress of the reaction (eluent; CCl4/Ether: 5/2). The reaction was filtered after completion within 5 min. Evaporation of the solvent and short column chromatography of the resulting crude material over silica gel (eluent; CCl4/Ether: 5/2) afforded the N-(3,7-dimethylocta-2,6-dienyl)benzenamine(0.l95 g, 85%).
Conclusion
In this investigation, we have shown that the combination reducing system of NaBH4/B(OH)3 in CH3CN can be used for reductive amination of citral with a variety of anilines to their corresponding N-citralbenzenaminesin good yields (75-80%) within 5 min at room temperature. Reduction reactions were carried out with 1 molar equivalents of NaBH4 in the presence of 1molar amounts of B(OH)3.
Acknowledgements
The authors gratefully appreciated the financial support of this work by Mohsen minaee.
References
- Varma, R. S.;Kabalka, G. W. Synth. Commun.,15: 155 (1985).
- Nutaitis, C. F.;Bernardo,J. E. J. Org. Chem.54: 5629 (1989).
- Krishnamurthy, S.; Brown, H. C.J. Org. Chem.40: 1864 (1975).
- Krishnamurthy, S.; Brown, H. C. J. Org. Chem.42: 1197(1977).
- Kim, S.; Moon, Y. C.;Ahn,and K. H.J. Org. Chem.47: 3311(1982).
- Setamdideh, D;.Khezri, B.;Rahmatollahzadeh, M.;Aliporamjad, A.Asian J. Chem. 24: 3591(2012).
- Mohamadi, M.; Setamdideh, D.; Khezri, B. Org. Chem. Inter., doi:10.1155/2013/127585 (2013).
- Setamdideh, D.; Hasani, S.; Noori, S. J. Chin. Chem. Soc.,60: 1267 (2013).

This work is licensed under a Creative Commons Attribution 4.0 International License.









