Comprative Infrared Study of cerium and thorium soaps
Ramakant Sharma
Department of Chemistry, Ambah P.G. Ambah Morena, India
DOI : http://dx.doi.org/10.13005/ojc/290352
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
Infrared spectra tests have shown that fatty acids exist with a dimeric structure through hydrogen bonding between two molecules of fatty acids whereas metal-to-oxygen bonds in metal soaps have an ionic character but the bonds are not purely ionic.
KEYWORDS:Infrared;Cerium;Thorium;soaps
Download this article as:| Copy the following to cite this article: Sharma R. Comprative Infrared Study of cerium and thorium soaps. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290352 |
| Copy the following to cite this URL: Sharma R. Comprative Infrared Study of cerium and thorium soaps. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=366 |
Intorduction
In spite of much work reported on alkali, alkaline earth and transition metal soaps, only few references [1-15] are available on tare earth metal soaps which have found wide application in industry. The present work was carried out with a view to investing the characteristics and structure of cerium and thorium soaps in the solid state by using infrared.
Experimental details
The fatty acids were purified by distillation under reduced pressure. Cerium and thorium (vi) soaps (laurate and myristate) were prepared the metathesis of an aqueous solution of cerium nitrate and thorium nitrate with a hot solution of the corresponding sodium soap. The precipitated soaps were filtered and washed with distilled water, alcohol and finally with acetone. The metal soaps thus obtained were first dried in an air oven and finally under reduces pressure and were further purified by recrystallization.
The melting points of purified cerium and thorium soaps were laurate 440c and myristate 540c. The soaps were analysed for carbon, hydrogen and metal contents and the result were found to be in agreement with the theoretically calculated values.
The infrared absorption spectra of lauric and myristic acids and their corresponding cerium and thorium soaps were determined using a Perkin-Elmer model 577 grating spectrophotometer in the region 4000-400 cm -1 using the potassium bromide disc method.
Result and Discussion
Infrared absorption spectra
The infrared spectral data of cerium and thorium soaps are listed in table 1,2,3,4 and compared with that of their corresponding fatty acids. The fatty acids (lauric and myristic acid) display a very broad intense peak due to –OH stretching near 2660-2650 cm-1 . The appearance of the absorption band near 1700-1680 cm-1 in the spectra of fatty acids indicates that the fatty acids exist as dimmers [16] .One of the characteristic bands of dimeric carboxylic acids from the out-of- Plane Bending –OH group appearing near 950-930 cm-1. The absorption maxima near 690 cm-1 and 550 cm-1in the spectra of fatty acids are associated with carboxyl group bending and wagging modes.
In cerium and thorium (iv) Soaps, two absorption bands are observed near 1440-1410 cm-1and 1610-1540 cm-1 instead of one strong absorption band corresponding to carboxyl group observed near 1700 cm-1 in the spectra of fatty acids. These bands correspond to symmetric and antisymmetric stretching vibrations of the carboxylate ion as pointed out by Duval, Lecomte and Douville [17]. The complete disappearance of the carboxyl frequency in the spectra of cerium and thorium soaps indicates that there is a complete resonance in the tow C-O bonds of the carboxyl group of the soap molecules. The metal –to- oxygen bond in cerium and thorium soaps in not purely ionic but is partially covalent in character. The bond observed at 440 cm-1 in the spectra of cerium and thorium soaps corresponds to the Ce-0,Th-O bond. The absorption bands observed near 2650,940,690 and 550 cm-1 which are associated with the carboxyl group of fatty acids, disappear completely in the spectra of cerium and thorium soaps.
The result confirm that the fatty acids in solid state exist with dimeric structure through hydrogen bonding between two molecules of fatty acids whereas metal-to-oxygen bonds in metal soaps are ionic in character but the bonds are not purely ionic.
 |
Table-l : Frequencies (cm–1) of Absorption maxima with their Assignments Click here to View table |
 |
Table-2 : Frequencies (cm–1) of Absorption maxima with their assignments Click here to View table |
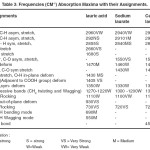 |
Table – 3 : Frequencies (CM-1) Absorption Maxima with their Assignments. Click here to View table |
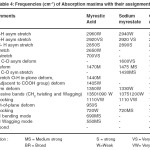 |
Table – 4 : Frequencies (CM-I) of Absorption maxima with their assignments. Click here to View table |
References
- K.N. Mehrotra, A.S. Gahlot and Meera Sharma: J. Am. Oil Chemists Soc. 63 (1986) 1571
- L.W. Rayan and W.W. Plechner: Ind. Eng. Chem.,26 (1934)909-10.
- H.W. Chatfield: Pa Manuf., 6(1936) 112-14.
- Titanium Pigment Co. Inc. Brit. 395-406, july 17 (1933).
- J. H. Skellon and J.W. Spence : J. Soc. Chem. Ind. London, 67 (1948)365-68.
- J.H. Skellon and J.W. Spence: J. Appl. Chem. London 3 (1953) 10-14.
- J.H. Skellon and K.E. Andrews:J. Appl. Chem. London,5(1955) 245-50
- G. Marwedal: Farbe u. lack 60 (1954) 530-37;62 (1956) 92-96.
- S.N. Misra, T.N. Misra and R.C. Mehrotra:J. Inorg. Nucl. Chem., 25(1963)195-199, 201-203.
- A.M. Bhandari, S. Dubey and R.N. Kapoor: J.Am. Oil Chemists’ Soc., 4 (1963) 47.
- R.C. Mehrotra: wiss. Z. Friedrich Schiller Univ., Jena Mitt. Naturwiss. 14(2) (1965) 171-80
- F.Mains, D. Mills and D.W. White: U.S.3, 320, 172, May 16 (1967)
- A.K. Solanki and A.M. Bhandari: Tenside Detergents. 18(1) (1981) 34-36.
- R.P. Varma and R. Jindal: Tenside Detergents, 20(4) (1983) 193-195
- L.D. Skrylev, V.F. Sazonna, M.E. Kornelli and N.A. Shumitina: Khim. Khim, Tekhnol. 21(4) (1987) 491-93.
- R.J. Dyer: “ Applications of absorption spectroscopy of organic compounds ‘’ Prentice hall of India Private Ltd. New Delhi, P. 46 (1969)
- C. Duval, J. Lecomte and F. Douville: Ann. Phys., 17(1942)5.
- R.D. Vold and G.S. Hattiangdi: Ind. Eng. Chem., 41 (1949)2311

This work is licensed under a Creative Commons Attribution 4.0 International License.









