Viscosity of Thorium Soaps
Ramakant Sharma and Meera Sharma*
Department of Chemistry, Agra College, Agra - 282 002, India.
The density and viscosity results of thorium soaps in benzene methanol mixture have been explained satisfactorily in terms of the equations proposed by Einstein, Vand and Jones- Dole. The values of the CMC and molar volume of thorium soaps calculated from these equations are in close agreement.
KEYWORDS:Thorium (II); Soaps; Viscosity
Download this article as:| Copy the following to cite this article: Sharma R, Sharma M. Viscosity of Thorium Soaps. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Sharma R, Sharma M. Viscosity of Thorium Soaps. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22236 |
Introduction
Metallic soaps are widely used in industries and allied sciences as catalysts, cosmetics, lubricants, greases, medicines, softeners, flatteners, stabilizers, plasticizers, emulsifiers, surface active agents and waterproofing agents. Therefore, a detailed study of these soaps is required for their great importance in industrial and academic fields. Extensive work has been done on the alkali, alkaline and transition metal soaps but comparatively less work has been done on lanthanide and actinide soaps1-4. Lanthanum soaps were prepared by Skrylev et al5 by the reaction of lanthanum chloride and corresponding salt of fatty acid. Mehrotra et al6 prepared the soaps of lanthanum, cerium, praseodymium and neodymium by double decomposition method. Skellon and Andrews7 studied the rate of oxidation of fatty acids in the presence of thorium soaps. Volatility and analysis of cerium soaps by treating with oxalic acid was studied by Marwedel8. Catalytic activity of cerium, thorium and uranyl soaps was studied by Skellon and Spence9 Physico-chemical studies, IR, X-ray and TGA, of thorium and lanthanum soaps in solid state was studied by Mehrotra et al10 Ultrasonic and conductivity studies of thorium soap solutions have beendone by Mehrotra et al11
The present work solutions have been of density and viscosity of thorium soap solutions in benzene-methanol mixture at different temperatures in order to examine their Micellar behavior and to check the validity of various known equations.
Experimental
All the chemicals used for the preparation of thorium soaps were of AR grade and were purified by standard methods. Thorium soaps were prepared by direct metathesis of the corresponding sodium soaps with the required amount of aqueous solution of thorium nitrate with constant stirring. The precipitated soap was filtered and washed first with distilled water and finally with alcohol. The metal soaps were first dried in an air oven and finally under reduced pressure and further purified by recrystallization.
The viscosity and density of the solutions of thorium soaps were measured by Ostwald’s Viscometer and Pyknometer at different temperatures.
Results and Discussion
Density
The density (r) of the solutions of thorium soaps Lourate and Myristate is determined at different temperatures (40, 50 and 60ºC). The density increases first rapidly and then slowly with the increase in the soap concentration and temperature. The plots of density vs. soap concentration (g mol dm-3) are characterized by an intersection of two straight lines at a definite soap concentration which corresponds to the critical micelle concentration (CMC) of the soaps, indicating a marked change in the aggregation of the soap molecules at the CMC. The values of the CMC decrease with the chain length of soaps but increase with temperature. The plots of density vs. soap concentration below the CMC have been extrapolated to zero soap concentration and the extrapolated values of the density, r0 are in agreement with the experimental values of the density of the solvent. The density results have been explained in terms of Root’s equations.
r = r0 + AC – BC3/2
Where C is the concentration of the soaps (g mol dm-3), r and r0 are the densities of the soap solution and solvent, respectively and the constants A and B refer to the solute-solvent and solute-solute interactions, respectively. The values of the constants A and B have been obtained from the intercept and slope of the plots of (r–r0)/C vs. C1/2 below the CMC. The results confirm that the soap-solvent interaction is larger than the solute-solute interaction in dilute soap solution. It is, therefore, concluded that there is a marked increase in the aggregation of the soap molecules and the soap molecules do not show appreciable aggregation below the CMC.
Viscosity
The viscosity (h) of the solutions of thorium soaps (Laurate and Myristate) in benzene-methanol mixture increases with the increase in the soap concentration and chain length of soap and decreases with temperature and vice versa. The plots of the viscosity h vs. the soap concentration are characterized by the intersection of two straight lines at a definite soap concentration which corresponds to the critical micelle concentration. The values of the CMC are affected by the chain length of the anion in the soap. The decrease in the CMC with the length of hydrocarbon chain and increase with temperature may be due to the increase in the stability of the micelles as well as due to the increase in the tendency of aggregation. The plots of the viscosity vs. soap concentration below the CMC have been extrapolated to zero soap concentration and it is observed that the extrapolated values of the viscosity for zero soap concentration are in agreement with the viscosity of pure solvents. This again confirms that the soap molecules do not aggregate to an appreciable extent below the CMC. The viscosity results are satisfactorily explained on the basis of the following equations.
Where, , C, f and hsp Are molar volume of the soap (dm3 mol-1), concentration of the soap (g mol dm-3), interaction coefficient and specific viscosity of the solution, respectively.
The values of the molar volume of the soap have been calculated from the slope of the plots of hsp vs. C (Einstein’s equation) and from the plots of 1/C vs. 1/[log (h/h0)] (Vand’s equation) and it is observed that the values obtained from both the equations are in agreement indicating that these equations are applicable to these soap solutions.
Table 1: Values Of Molar Volume, and Constants A and B Obtained From Different-Equations at Different Temperatures
| Soap | Einstein’s eqn. | Vand’s eqn. | Jones-Dole’s eqn. | Root’s eqn. | ||
| x 10-2 dm3 (mol-1) | A | B x 10-2 | A | B x 10-2 | ||
| Temperature 40ºC | ||||||
| Laurate | 3.13 | 3.07 | 1.00 | 7.50 | 23.00 | 3.00 |
| Myristate | 3.20 | 3.29 | 1.25 | 7.69 | 23.50 | 3.13 |
| Temperature 50º | ||||||
| Laurate | 2.75 | 2.76 | 0.75 | 6.67 | 29.75 | 2.50 |
| Myristate | 2.91 | 2.93 | 1.00 | 6.78 | 30.25 | 2.73 |
| Temperature 60º | ||||||
| Laurate | 3.00 | 3.15 | 0.25 | 6.00 | 40.25 | 2.50 |
| Myristate | 3.20 | 3.22 | 0.75 | 6.11 | 35.25 | 2.85 |
Table 2: Values Of The Cmc (G Mol Dm-3), C X 104
| Soap | Temperatures (ºC) | ||
| 40 | 50 | 60 | |
| Thorium Laurate | 6.10 | 6.30 | 6.35 |
| Thorium Myristate | 6.00 | 6.20 | 6.30 |
| Conc. Of Soap, CX104(g mol dm-3) | Vicosity at different temperatures (0C) | ||
| 40 | 50 | 60 | |
| Thorium Laurate | |||
| 1.0 | 5.22 | 5.16 | 5.12 |
| 2.0 | 5.58 | 5.47 | 5.40 |
| 3.0 | 5.96 | 5.82 | 5.64 |
| 4.0 | 6.34 | 6.13 | 5.99 |
| 5.0 | 6.74 | 6.39 | 6.20 |
| 6.0 | 7.12 | 6.77 | 6.52 |
| 7.0 | 7.60 | 7.21 | 6.89 |
| 8.0 | 8.11 | 7.73 | 7.40 |
| 9.0 | 8.62 | 8.67 | 7.85 |
| 10.0 | 9.00 | 8.62 | 8.34 |
| Thorium Myristate | |||
| 1.0 | 5.21 | 5.14 | 5.09 |
| 2.0 | 5.60 | 5.47 | 5.34 |
| 3.0 | 5.97 | 5.84 | 5.62 |
| 4.0 | 6.40 | 6.21 | 5.95 |
| 5.0 | 6.73 | 6.50 | 6.21 |
| 6.0 | 7.12 | 6.88 | 6.48 |
| 7.0 | 7.64 | 7.35 | 6.90 |
| 8.0 | 8.11 | 7.89 | 7.44 |
| 9.0 | 8.64 | 8.34 | 7.98 |
| 10.0 | 9.10 | 9.01 |
8.47 |
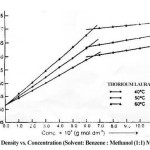 |
Figure 1 Click here to View figure |
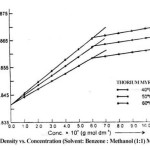 |
Figure 2 Click here to View figure |
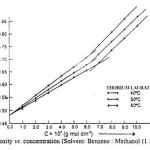 |
Figure 3 Click here to View figure |
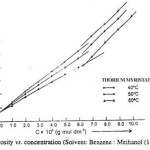 |
Figure 4 Click here to View figure |
The applicability of Jones-Dole’s equation was checked by the plots of hsp/C12 vs. C1/2 which are linear below the CMC. The values of coefficients A and B are calculated from the intercept and slope of the plots below the CMC. The values of B are larger than the values of coefficient A, which confirms that the molecules of the soap do not aggregate appreciably below the CMC and there is sulden change in the aggregation above the CMC.
It is, therefore, concluded that the equations of Einstein, Vand and Jones-Dole are applicable to dilute solutions of thorium soaps in benzene-methanol mixture. The values of the CMC and molar volume of thorium soaps calculate from these equations are in close agreement.
References
- K.N. Mehrotra, A.S. Gahlaut and M. Sharma, J. Am. Oil Chem. Soc., 63, 157 (1986).
- R.P. Varma and R. Jindal, Tenside Detergents, 20, 193 (1983).
- W. Brzyska and W. Hubicki, Ann. Univ. Maide Curie Sklodowksa Soc.,(A/P), 24/25, 69 (1970).
- A.M. Bhandari, S. Dubey and R.N. Kapoor, J. Am. Oil Chem. Soc., 47, 47 (1990).
- L.D. Skrylev, V.F. Sazonoma, M.E. Kornelli and N.A. Shukiline, Khim. Khim. I khnol., 21, 491 (1978).
- R.C. Mehrotra, Wizz. Z. Friedrich-Schillan Univ. Jena, Math. Naturewise Ket., 14,, 171 (1965).
- .H. Skellon and K.E. Andrews, J. Appl. Chem. (London), 5, 245 (1955).
- G. Marwedel, Farbe U. Lack, 60, 530 (1954); 62, 92 (1956).
- J.H. Skellon and J.W. Spence, J. Appl. Chem. (London), 3, 10 (1953).
- K.N. Mehrotra, A.S. Gahlaut and M. Sharma, J. Indian Chem. Soc., 65, 397 (1988).
- J. Indian Chem. Soc., 64, 729 (1987); Acoustics Lett. 12, 107 (1988); Recl. Trav. Pays Bas., 107, 310 (1988); J. Phy. Chem. Liq., 20, 147 (1988).
- A. Einstein, Ann. Phys., 19, 289 (1906).
- V. Vand. J. Phys. Colloid Chem., 52, 277 (1948).
- G. Jones and M. Dole, J. Am. Chem. Soc., 51, 2950 (1929).

This work is licensed under a Creative Commons Attribution 4.0 International License.










