The Adsorption Equilibrium Isotherms Freundlich, Langmuir, and Temkin Models for Two Steroidal anti-Inflammatory Drugs (NSAIDs) Betamethasone and Clobetasol on Multi-Walled Carbon Nanotube
Mehdi Vadi*, Najaf Hossinie and Zahra shekari
Department of Chemistry, Gachsaran Branch, University of Gachsaran, Kohgiloyeh va Boyerahmad, Iran.
Corresponding Author E-mail: mahdi_vadi@iaufasa.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/290242
We have studied the interaction of Betamethasone and Clobetasol solutions on multi carbon nanotube. After investigated comparative study and assigned to either Betamethasone or Clobetasolon adsorption isotherms. The adsorption equilibrium isotherms were fitted by Freundlich, Langmuir, and Temkin models. It was found that the Langmuir model described the adsorption process better than other two isotherm models. The amount of SAIDs (Betamethasone, Clobetasol) adsorbed on carbon nanotube surface increased with the increase of the initial SAIDs concentration. The results show that Clobetasolon has most amount adsorption rate of Multi walled carbon nanotubes (MWCNTs).
KEYWORDS:Adsorption; Betamethasone; Clobetasol; Multi-walled Carbon nanotubes
Download this article as:| Copy the following to cite this article: Vadi M, Hossinie N, Shekari Z. The Adsorption Equilibrium Isotherms Freundlich, Langmuir, and Temkin Models for Two Steroidal anti-Inflammatory Drugs (NSAIDs) Betamethasone and Clobetasol on Multi-Walled Carbon Nanotube. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Vadi M, Hossinie N, Shekari Z. The Adsorption Equilibrium Isotherms Freundlich, Langmuir, and Temkin Models for Two Steroidal anti-Inflammatory Drugs (NSAIDs) Betamethasone and Clobetasol on Multi-Walled Carbon Nanotube. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22211 |
Introduction
Carbon nanotubes (CNTs) are allotropes of carbon with a cylindrical nano structure.. Nanotubes have been constructed with length to diameter ratio of up to 132,000,0001.Significantly larger than for any other material. These cylindrical carbon molecules have unusual properties, which are valuable for nanotechnology, electronics, optics and other fields of materials science and technology. In particular, owing to their extraordinary thermal conductivity and mechanical and electrical properties, carbon nanotubes find applications as additive to various structural materials. For instance, nanotubes from only a tiny portion of the material(s) in (primarily carbon fiber) baseball best, golf clubs, or car parts2 nanotubes are members of the fullerene structural family, which also includes the spherical buckyballs , and the ends of a nanotube may be capped with a hemisphere of the buckyball structure.Their name is derived from their long, hollow structure with the walls formed by one atom thick sheets of carbon, called graphen3. These sheets are rolled at specific and discrete (chiral) angles and the combination of the rolling angel and radius decides the nanotubes properties; for example, whether the individual nanotubes shell is a metal or semiconductor. Nanotubes are categorized as single walled nano tubes (SWNTs) and multi walled nanotubes (MWNTs)4. Individual nanotubes naturally align themselves into ropes held together by vanderwaals forces, more specifically, orbital hybridization best describes chemical bonding in nanotubes5,6. The chemical bonding of nanotubes is composed entirely of sp2bonds, similar to those of graphite. These bonds, which are stronger then the sp3 bonds found in alkanes and diamond, provide nanotubes with theirunique strength7. Multi walled nanotubes (MWNT) consist of multiple rolled layers (concentric tubes) of graphene. There are two model, sheets of graphite are arranged in concentric cylinders, e.g, a (0.8) single walled nanotube (SWNT) within a larger (0.17) single-wallednanotube8. steroidal anti-inflammatory drugs, usually abbreviated to SAIDs but also referred to as steroidal anti-inflammatory agents/analgesics (SAIAs) or steroidal anti-inflammatory medicines (SAIMs) are drugs that analgesic and antipyretic fever-reducing) effects and in higher doses, anti-inflammatory effects9. The term “steroidal” distinguishes these drugs from steroids, which, among a broad range of other effects, have a similar anti-inflammatory action10-28.
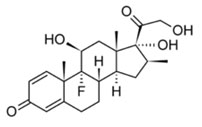
Betamethasone Formula
Betamethasone is the protype member of a large family of steroids anti-inflammatorydrug (SAID).Designated chemically as (8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro- 11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl- 6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro- 3H-cyclopenta[a]phenanthren-3-one.Betamethasone use situation treatmentfor adrenal insufficiency ,so These drugs symptomatic treatment of inflammatory disorders and sensitivity in order to suppress the system safety. The molecular weight is 392.461g/mol. Its molecular formula is C22H29FO5 . The structural formula is:
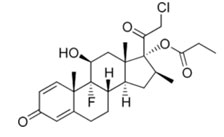
Clobetasol Formula
Clobetasol is the pro type member of a large family of steroids anti-inflammatory drug (SAID).Designated chemically as (11β)-11, 17, 21-trihydroxypregn-4-ene-3, 20-dione.These drugs used in human medicine and exhibit a wide range of topical potencies. The molecular weight is 466.97g/mol. Its molecular formula is C25H32ClFO5. The structural formula is:
Material
We used the carbon nanotube with 95%pure degree, production of neutrino company.Betamethasone and Clobetasol with 99% pure degree production of Pharmaceutical companies Aboureihan.
Apparatus
In this survey the spectrophotometer (Jenway6505, Model, England), magnetic stirrer (Heidolph, Mr3001 Model), Analytical balances (Sartorius Model), Filter paper (Albet), were used.
Adsorption experiments
In order to provide Betamethasone and Clobetasol solution, twice distilled water was used .multi walled carbon nanotube was used as an adsorbent. First 50ppm of Betamethasone and Clobetasol was provided using this sample, some solutions with different concentrations of 4to 12mg/lit of pure Betamethasone and Clobetasol were prepared specific amount of carbon nanotube (0.005gr) was added to flasks containing Betamethasoneand Clobetasol,as an adsorbent .it was stirred , using a magnetic stirrer for 10 minutes. Then liquid and solid phase were separated by means of a filter paper. The concentration of Betamethasoneand Clobetasolwas measuredby using on spectrophotometer tool adsorption rate, gained for SAIDs .All test have been performed at the lab with the temperature.
Adsorption equilibrium isotherms
In a two component system consisting of absorber solution, a graph of the concentration of dissolved solid phase qe (mg / g) was dissolved in the solution concentration Ce (mg/L) in an equilibrium adsorption isotherm is expressed. In a liquid-solid adsorption from solution onto the surface of these parathion of dissolved solids remaining dissolved in the solution until the solution reaches the dynamic equilibrium on a solid surface.A distributed constraint solving in equilibrium between liquid and solid phases which may be explained by a number of isotherms Adsorption model scan be found for the correlation between experimental data and model parameters. Several mathematical models can be used to describe experimental data of adsorption isotherms. The Freundlich, Langmuir and Temkin models are employed to analysis adsorption occurred in the experiment. Data of adsorption isotherms. The Freundlich, Langmuir and Temkin models are employed to analysis adsorption occurred in the experiment. Adsorption isotherms to describe the adsorption capacity to facilitate the process of evaluating the feasibility of the intended application and is useful for the analysis and design of adsorption systems.
Langmuir model
The Langmuir adsorption model is the most commonly used model of adsorption, which is described by the following equation:
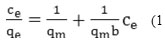
In this equation, qe (mg / g) is the solution was adsorbed the surface and qe is equilibrium constant of adsorption and b is the capacity of adsorption in saturated single layer and Ce (mg/L) Is solution in equilibrium state.
Freundlich model
Isotherm is an empirical equation for understanding the adsorption of ions on the surface of a heterogeneous multi-layer and the amount of dissolved in definitely by increasing the concentration of the high. Freundlich adsorption isotherm is described by the following equation:
![]()
In this equation, qe (mg / g) is amount of absorbed material in absorbent surface, K; n in arrangement are adsorption capacity and adsorption intensification.
Temkin model
ccompliance isotherm contains a factor of the interaction between the adsorbent particles adsorb clearly shows. Isothermal compliances achieved as follows:
![]()
Where A and B are the Temkin isotherm constant (L/g) and heat of sorption (J/mol) respectively. R is the gas constant (J/mol/k), b is the Temkin isotherm constant linked to the energy parameter, B, as shown on equation:
b = RT/B (4)
T is the absolute temperature in Kelvin.
Results and discussion
The Langmuir, Freundlich and Temkin isotherms of the adsorption process of SAIDs on CNT are shown in figures 1 to 3 and calculated parameters of these models are shown in Table 1. It was observed that the experimental data were well represented by Langmuir, Freundlich and Temkin models.
Table 1: Parameters of Langmuir, Freundlich and Temkin isotherms of the Betamethasone and Clobetasol on CNT
|
|
Langmuir |
|
Freundlich |
|
Temkin |
|
||||||
|
b |
q |
|
n |
|
B |
|||||||
|
Betamethasone |
1.94 |
2.178 |
0.9906 |
0.70 |
5.08 |
0.999 |
1.36 |
12.99 |
0.9922 |
|||
|
Clobetasol |
0.22 |
53.19 |
0.9571 |
1.227 |
9.72 |
0.998 |
3.522 |
7.877 |
0.99 |
|||
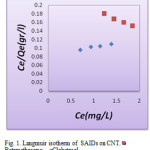 |
Figure 1. Langmuir isotherm of SAIDs on CNT. Click here to View fidure |
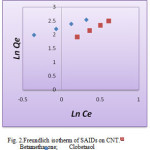 |
Figure 2: Freundlich isotherm of SAIDs on CNT. Click here to View figur |
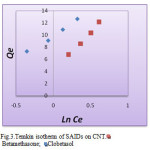 |
Figure 3: Temkin isotherm of SAIDs on CNT. Betamethasone; Click here to View figure |
Betamethasone; Clobetasol Betamethasone; Clobetasol
Conclusions
This study compares the adsorption isotherms of some steroid drugs were studied. Principles of adsorption spectroscopy are that the number of molecules absorbing certain wave lengths of light, the more is absorbed.224-280nm range are absorbed steroids. On-bond electrons by the resonance of the π system increases. What ever the aromatic ring system of access to the electrons is greater than the shift linkage would be more. Depending on the nature of the electron donor and electron attraction of the substituent groups are capable of absorbing a variety of situations. In this study,then umber of functional groups and double bonds steroid medications increases the adsorption rate is higher. With increasing concentration of is adsorption more, it can be looked at whatever level of involvement with drugs (SAIDs) are the most adsorbent of carbon nanotubes and multi-wall adsorbed dose steroids are also higher.
in this research, adsorption some of steroidal anti-inflammatory drugs (Betamethasone and Clobetasol) on multiwall carbon nanotube from aqueous solution were studied. The results show that the amount of Betamethasone and Clobetasol adsorbed on carbon nanotube surface increased with the increase of the initial SAIDs concentration, so this results show that depending on the nature of the electron donor and electron attraction of the substituent groups are capable of absorbing a variety of situations. In this study, the number of functional groups and double bonds steroid medications increases the adsorption rate is higher. The experimental results show Clobetasol has most amount adsorption rate of MWCNT it can be attributed to lower the barrier of space in this material and that it was found that the Freundlich model described the adsorption process better than other two isotherm models.
References
- Wang, X.; Li, Qunqing; Xie, Jing; Jin, Zhong; Wang, Jinyon Li, Yan; Jiang, Kaili;Fan, Shoushan “Fabrication of Ultralong and Electrically Unifor Single-Walled Carbon Nanotubes on Clean Substrates. Nano Letters 9 (9):3137-3141,(2009).
- Single-Walled Carbon Nanotubes on Clean Substrates .Nanotechnology: A Guide to Nano- Objects.Chemical Engineering Progress 107 (5): 28–32.
- Flahaut, E.; Bacsa, Revathi; Peigney, Alain; Laurent, Christophe “Gram-Scale CCVD Synthesis of Double-Walled Carbon Nanotubes”.Chemical Communications 12 (12): 1442–1443, (2003)
- Cumings, J.; Zettl, A. “Low-Friction Nanoscale Linear Bearing Realized from Multiwall Carbon Nanotubes”. Science 289 (5479): 602–604, (2000)
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. “Exceptionally high Young’s modulus observed for individual carbon nanotubes”. Nature 381 (6584): 678–680, (1996)
- Zavalniuk, V.; Marchenko, S. “Theoretical analysis of telescopic oscillations in multi-walled carbon nanotubes”. Low Temperature Physics 37 (4). 337, (2011)
- F.martines,A.hernance,protein,Adsorption ontonictofiltrationmemberance., interaction 254-2612 (2006).
- W. A. De Heer, W. S. Bacsa, A. Chatelain, T. Gerfin, R. Humphrey Baker, Nanocapillarity and chemistry in carbon nanotubes, Science 268, 1995, Page 845..
- Simone Rossi, ed. Australian medicines handbook 2006.Adelaide: Australian Medicines Handbook Pty Ltd.ISBN 0-9757919-2-3, (2006)
- Gøtzsche, Pc “Methodology and overt and hidden bias in reports of 196 double-blind trials of steroidal anti-inflammatory drugs in rheumatoid arthritis”. Controlled clinical trials 10 (1): 31–56,(1989)
- Anonymous Cancer pain relief and palliative care; report of a who expert committee. World Health Organization Technical Report Series, 804. Geneva, Switzerland: World Health Organization. pp. 1-75, (1990).
- M.Vadi, F.Tolou.”Study of the Frondlich, Langmuir and Temkin, adsorption isotherms for some amino acids and their complexation with Lanthanum (III) on multi-walled-carbon nanotube”. Oriental Journal of Chemistry. Vol.27 (2).545-551
- M.Vadi, Y.Ghasemi.”Adsorption isotherms 2-amino phenol and 4-amino phenol on carbon nanotube”. Oriental Journal of Chemistry.Vol.27 (2).563-566
- M.Vadi, M.Zakeri.”Thermodynamic study of Chrysens adsorption by multi-walled on carbon nanotube”. Oriental Journal of Chemistry.Vol.27 (3).945-951
- M.Vadi, E.Ghaseminejhad.”Comparative study of isotherms adsorption of Oleic acid by activated carbon and multi-wall carbon nanotube” Oriental Journal of Chemistry.Vol.27(3).973-978
- M.Vadi.”Adsorption isotherm study of vitamin B2 on carbon nanotube”.Oriental Journal of Chemistry.Vol.27 (3).1037-1040
- M.Vadi, N.Moradi.”Study of adsorption isotherms of Acetamide and Propionamide on carbon nanotube” Oriental Journal of Chemistry.Vol.27 (4).1491-1495
- M.Vadi, F.Pooladian.”Examination of surface adsorption isotherms of the complex of some amino acid with Cr (III) ion by carbon nanotube”. Oriental Journal of Chemistry.Vol.27 (4).1517-1521
- M.Vadi.”Comparative study of adsorption isotherms two non- steroidal anti- inflammatory eye drops, Indomethacin and Diclofenac on carbon nanotube” Oriental Journal of Chemistry.Vol.28(1).343-348
- M.Vadi, Y.Noshadi.” Investigation of adsorption isotherms of Oxymethalone as a kind of steroid drug by multi-wall carbon nanotube “Oriental Journal of Chemistry.Vol.28 (1).297-301
- M.Vadi, A.Lorestani.”Adsorption isotherms of Diethyl and Dimethyl malonate esters on single-walled carbon nanotubes “Oriental Journal of Chemistry.Vol.28 (3).1195-1199
- M.Vadi, O.Bazipour, A. Malekzadeh.”Adsorption isotherms study of Ketotifen as a drug on multi- walled carbon nanotube”. Oriental Journal of Chemistry.Vol.28 (3).1285-1289
- M.Vadi, O.Bazipour , A. Malekzadeh.”Evaluation of adsorption isotherms of Frenulich, Temkin and Langmuir of Loratadine drug on multi-walled carbon nanotube” Oriental Journal of Chemistry.Vol.28(3).1305-1309
- M.Vadi, A.Omidi.” Comparative study of adsorption isotherms two non- steroidal anti- inflammatory drugs Acetaminophen and Diclofenac on carbon nanotube”. Oriental Journal of Chemistry.Vol.28(3).1325-1330
- M.Vadi, A. Maleki, F. Polladiyan,”Adorption Some Alkaline Metals and Alkaline Earths Metals on Carbon Nanotube: Asian Journal of Chemistry .Vol.22 (9).6906-6910
- M.Vadi, M. Abbasi ,“Adsorption Isotherms Complexation of Some Amino Acids with Co(II) Ion on Carbon Nanotube”. Asian Journal of Chemistry .Vol.23 (8). 3431 – 3434
- M.Vadi, F Saybankhirabadi, “Investigation of Adsorption Isotherms of 2-Naphthol on Carbon Nanotube” Asian Journal of Chemistry .Vol.24 (4). 1618 – 1620
- M.Vadi, “Adsorption Isotherms of Some Non-Steroidal Drugs on Single Wall Carbon Nanotube” Asian Journal of Chemistry .Vol.25 (6). 3431 – 3433

This work is licensed under a Creative Commons Attribution 4.0 International License.









