Synthesis of New Oxidant N-chloroisonipecotamide and its Oxidation Kinetics on Aromatic Acetals
K. Shenbagam1 and N. Mathiyalagan2
1M.A.M. College of Engineering, Siruganur, Tiruchirappalli - 621 105, India.
2Jayaram College of Engineering and Technology, Thuraiyur (Tk), Tiruchirappalli - 621 014, India.
Corresponding Autohr E-mail: senba_5@rediffmail.com
The new oxidant N-chloroisonipecotamide (NCINA) is prepared by standard procedure and characterized. The formal redox potential, physical constant, elemental analysis and spectral characterization (IR, 1H-NMR and Mass spectrum) confirms the presence of nitrogen-halogen bond. It is found to be a mild and stable oxidant and formal redox potential of N-chloroisonipecotamide shows that it can be used as an effective source of positive halogen, like other existing oxidants. Oxidation of benzaldehyde di-n-alkyl acetals is carried out with new oxidant in acetonitrile medium. The reaction is first order with respect to NCINA and zero order with respect to acetals. The effect of isonipecotamide and acetonitrile is studied. Increase in ionic strength has marginal effect on the rate. A mechanism consistent with the observed kinetic results has been elucidated.
KEYWORDS:Synthesis; N-chloroisonipecotamide; Kinetics; Oxidation; Acetal
Download this article as:| Copy the following to cite this article: Shenbagam K, Mathiyalagan N. Synthesis of New Oxidant N-chloroisonipecotamide and its Oxidation Kinetics on Aromatic Acetals. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Shenbagam K, Mathiyalagan N. Synthesis of New Oxidant N-chloroisonipecotamide and its Oxidation Kinetics on Aromatic Acetals. Orient J Chem 2013;29(2).Available from: http://www.orientjchem.org/?p=22244 |
Introduction
Oxidation is an important process in organic chemistry and us of a new economic and effective oxidants under mild and anhydrous conditions constitutes a standing challenge. The chemistry of N-halo compound forms a separate branch, which is a great synthetic importance1. N-halo compounds have been extensively employed as oxidizing agents for organic substrates2. The electronegativities of halogens except fluorine are less than that of nitrogen. Hence they acquire a positive charge when linked with nitrogen. The electronegativity of nitrogen is further enhanced by linking it with certain electron withdrawing groups such as acyl group. Thus N-halo compounds are referred as positive halogen compounds. Some of the N-halo compounds are N-bromonicotinamide, chloramine –T, N-bromoisonicotinamide, N-chloroisonicotinamide, N-chloronicotinamide, bromamine –T etc. They act as sources of positive halogens. N-chloro compounds have been used as oxidants in kinetic studies for the oxidation of various substartes3. N-halo reagents has been used as catalysts, in various chemical transformations, for the protection of functional groups etc. N-haloamides are being used as photoinitiators for radical polymerization, in room temperature for the preparation of caulks and sealants4 and as oxidizing agent in aqueous and non-aqueous media5,6. This diverse nature of N-halo compounds attracted and prompted to search a new oxidant. And oxidation kinetics of acetals is carried out with the new oxidant to prove the potential of the new oxidant. Acetals are synthetically useful gem-dialkoxy compounds. The oxidation of aliphatic and aromatic acetals with several oxidants7-13 has been reported and number of reports on the oxidation of several substrates by N-chloroamides is available in literature14-18. However the biological significance and versatile nature of isonipecotamide prompted to study the title investigation to throw more light on mechanistic aspects.
Materials and methods
Many literature methods are reported for the synthesis of primary N-chloroamides19 and secondary N-chloroamides20 using various chlorinating agents. Trichloroisocyanuric acid is one such chlorinating agent and has been demonstrated to improve the preparative efficacy and to dramatically reduce retention times for many organic transformations21. Colourless crystals of N-chloroisonipecotamide were prepared by the method reported in literature22. In a 50 mL round bottom flask were placed in ten mL methanol and added 3.22 m mol of isonipecotamide (INA) and the mixture was stirred until the solid dissolved. About 1.18 m mol (3.55 meq) of TCICA was added and cyanuric acid was precipitated. After stirring for one h, the mixture was filtered and the solid was washed with methylene chloride. The solvent removal by a rotary evaporator gave N-chloroisonipecotamide (90% yield); mp 189-190 0C; the chloramide (NCINA) precipitated was recrystallized with ether.
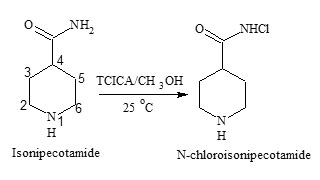
The purity of NCINA (Fig.1) was confirmed by elemental analysis (C 44.30 %, H 6.76%, N 14.76%, Cl 21.84 %, and O 9.84%). The IR spectra of NCINA showed the presence of secondary amide (1650 cm-1), a carbonyl group (1430 cm-1) and N-Cl band (1140 cm-1). The chemical shift found in the 1H NMR signals for both INA and NCINA was found to be the same and the peak corresponding to NH2 signal is slightly shifted to a larger value while the integration of the peak was half. These facts indicates that one proton of the NH2 group is substituted by the chlorine atom. 1H NMR of isonipecotamide δ: 7.3 (singlet due to –NH), 2.9 (singlet due to –CONH2), 2.1 and 2.4 (C5-H and C3-H, doublets of triplet due to equatorial and axial hydrogen interaction), 1.6 and 1.4 (C2H and C6H, multiplets, due to axial and equatorial hydrogen interaction). The signal remains the same for N-chloroiosnipecotamide and the peak corresponding to –CONH was slightly shifted to δ 7.6. Further the compound has been confirmed by mass spectrum which shows parent ion peak at m/z: 163 and M+2 peak at 165. Formation of peak at (M+2) also confirms the presence of chlorine in the molecule.
NCINA was found to be soluble in water, acetic acid, ethanol, but partially soluble in DMSO, DMF, CCl4, ethyl acetate, chlorobenzene and dioxane. Stock solution of NCINA was prepared in 80% acetic acid mixture and kept in the amber colored bottle. It was stable in appearance and concentration over a period of three months. A digital potentiometer (Equip-tronics dual channel potentiometer EQ-603) with a smooth platinum electrode and a saturated aqueous calomel electrode was used to determine the formal redox potential of NCINA and INA in 80% acetic acid and 0.1 M HCl. Dilute solutions of INA and NCINA were used and hence activities were replaced by concentrations terms in the Nernst equation.
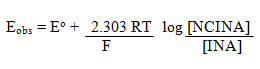
A plot of Eobs against log [NCINA]/[INA] yield a straight line with non-zero intercept (Figure.1). The potential was found to be 1.087 V at 25 oC. It shows that it is a strong oxidizing agent. The formal redox potential of the [NCINA]/[INA] couple viz. 1.087 V for N-chloroisonipecotamide at 25 oC is comparable to the value of 1.16 V for bromamine-T23, 1.05 V for N-chloroisonicotinamide24, 0.797 V for N-bromonicotinamide25, 0.808 V for N-bromoisonicotinamide26 and 1.02 V for N-chloronicotinamide27.
Acetonitrile (A.R grade) was purified by the literature procedure28. Benzaldehyde di-n-alkyl acetals namely benzaldehyde di-ethyl acetal, benzaldehyde di-n-propyl acetal, benzaldehyde di-n-butyl acetal, benzaldehyde di-isobutyl acetal, benzaldehyde di-isoamyl acetal and benzaldehyde dibenzyl acetal were prepared in the laboratory and their purities were checked by usual methods. Double distilled water was employed in all kinetic runs.
All kinetic measurements were made at pseudo-first order conditions, by keeping large excess of [acetal] over [oxidant]. Mixture containing requisite amounts of solutions of acetal, sodium perchlorate and acetonitrile were equilibrated at 323 K. To this mixture was added a measured amount of pre-equilibrated standard solution of NCINA. To maintain the desired temperature (± 0.1 °C) the reaction mixture was kept in a thermostated water bath and the progress of the reaction was monitored potentiometrically by setting up a cell consisting of a redox electrode (platinum wire was dipped in reaction mixture) and reference electrode (saturated calomel electrode). The emf of the cell was measured periodically using an equip-tronics (EQ-DGD) potentiometer.
Stoichiometry and product analysis
Stoichiometry of the reaction was ascertained by equilibrating the reaction mixture containing an excess of [NCINA] over [acetal], sodium perchlorate and acetonitrile for 24 h at room temperature. The concentration of unreacted oxidant was determined iodometrically. The estimated amount of unreacted NCINA showed that one mole of acetal consumed one mole of NCINA.
![]()
The reaction mixture containing excess of [acetal] over [NCINA] was kept overnight. The products in the reaction mixture were extracted several times with water followed by diethyl ether. The combined ether extracts was evaporated and the product ester was identified by the TLC, IR and 1H-NMR spectral data.
Results and discussion
With the [acetal] in excess, at constant [NaClO4] and temperature, plots of log [NCINA] vs time were linear (r > 0.99) indicating a first order dependence of rate on [NCINA]0. The values of pseudo-first order rate constants (kobs) are given in Table-1. Further the values of kobs, are unaffected with variation of [NCINA]0, confirming first order dependence on [NCINA]0. The rate remains unaltered with increase in [acetal] (Table-2), indicating a zero order dependence of rate on [acetal]. The corresponding rate constants with increase in [acetal] are given for benzaldehyde di-n-butyl acetal as a representative data in Table-1. Addition of halide ion in the form of NaCl or variation of ionic strength of the medium using NaClO4 showed only marginal effect on the rate. The increases in dielectric constant of the medium by adding water (0-8%), decrease the rate. On adding a small amount of the reaction mixture to acrylonitrile, there was no polymerization indicating the absence of free radical species. The reaction was carried out in the range of temperature 318 K to 333 K, keeping other experimental conditions constant. The values are given in Table-3. From the linear Arrhenius plot log kobs vs 1/T (r = 0.99), values of composite activation parameters Ea, ∆H#, ∆S# and ∆G# were calculated.
Mechanism
The oxidizing species in NCINA may be HOCl, H2OCl+, cationic chlorine and NCINA itself as expected for other N-chloroamides. NCINA, if taken as active species, it is unable to explain the slight negative effect of isonipecotamide on the reaction rate. If addition of chloride ions increases the rate of oxidation, Orton rearrangement of organic haloamides would have been expected. However no such effect was noticed. Since the rate is independent of ionic strength H2OCl+ and Cl+ can be ruled out. The hydrolysis of NCINA is slight and if HOCl is primarily involved, a retardation of rate by the added isonipecotamide is expected. Such effect is also noticed and hence it might be the active oxidant species.
Based on the above observations, the most probable mechanism is proposed as follows.
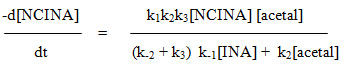
R= alkyl substituent [R= ethyl, n-propyl, n-butyl, isobutyl, isoamyl, benzyl].
Considering the above steps the rate of oxidation of acetal may be expressed in terms of change in [NCINA]. On applying steady state approximation to [HOCl] and [complex], the rate law is derived as follows.
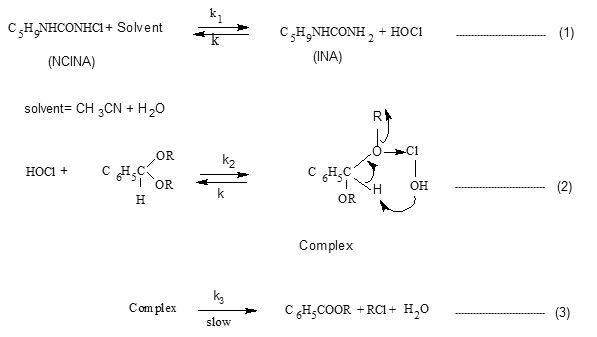
Since the retarding effect of [INA] is negligible on rate, it can be assumed that k-1[INA] << k2 [acetal] and the term k-1[INA] is ignored.
Hence the rate law reduces to
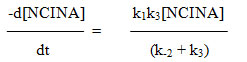
The above rate law explains the kinetic results with respect to [NCINA] and [acetal]. The above mechanism is well supported by Deno Potter29, Gopalakrishnan30 and is evidenced in the oxidation of acetals by NCN31.
The effect of solvent on the reaction rate has been studied by Amis32. In the present investigation a plot of log kobs vs % CH3CN is linear with positive slope. The negative value of entropy of activation as observed suggests that the activated complex is more compact than the reactants. High positive values of free energy of activation and enthalpy of activation suggest that transition state is highly solvated and similar mechanisms are operative for the oxidation of all the above mentioned aromatic acetals by NCINA in acetonitrile medium.
![Figure 1: Determination of Eo[NCINA]/[INA] potentiometrically in a mixture of 0.1 M HCl and 80% acetic acid - 20 % H2O (v/v) at 25 oC.](http://www.orientjchem.org/wp-content/uploads/2013/06/Vol33No4_Synt_Shriv_fig12-150x150.jpg) |
Figure 1: Determination of Eo[NCINA]/[INA] potentiometrically in a mixture of 0.1 M HCl and 80% acetic acid – 20 % H2O (v/v) at 25 oC. Click here to View figure |
Table 1. Effect of variation of [acetal] and [NCINA] on the rate constant for the oxidation of benzaldehyde di-n-butyl acetal by NCINA at 323 K.
[NaClO4] = 1.0 x 10-1 M CH3CN = 100 %
|
[NCINA] x 103 (M) |
[Benzaldehyde di-n-butyl acetal] x 102 (M) |
kobs x 104 (s-1) |
|
5.0 |
5.0 |
8.29 |
|
6.0 |
5.0 |
8.26 |
|
7.0 |
5.0 |
8.29 |
|
8.0 |
5.0 |
8.28 |
|
9.0 |
5.0 |
8.27 |
|
5.0 |
5.0 |
8.29 |
|
5.0 |
6.0 |
8.31 |
|
5.0 |
7.0 |
8.34 |
|
5.0 |
8.0 |
8.39 |
|
5.0 |
9.0 |
8.41 |
Table 2. Effect of solvent composition on the rate constant for the oxidation of benzaldehyde di-n-alkyl acetals by NCINA.
[C6H5CH(OC4H9)2] = 5.0 x 10-2 M [NCINA] = 5.0 x 10-3 M
[NaClO4] = 1.0 x 10-1 M
|
CH3-CN–H2O ( %) |
kobs x 104 (s-1) |
|||||
|
R = ethyl |
R = n- propyl |
R = n-butyl |
R = isobutyl |
R = isoamyl |
R = benzyl |
|
|
100-0 |
8.14 |
8.19 |
8.29 |
8.32 |
8.37 |
8.46 |
|
98-2 |
7.26 |
7.45 |
7.63 |
7.12 |
7.14 |
7.76 |
|
96-4 |
6.10 |
6.43 |
6.71 |
6.42 |
6.65 |
6.97 |
|
94-6 |
4.72 |
4.96 |
5.03 |
5.21 |
5.28 |
5.93 |
|
92-8 |
3.99 |
4.12 |
4.27 |
4.53 |
4.71 |
5.33 |
Table 3. Temperature dependent rate constant and activation parameters for the oxidation of benzaldehyed di-n-alkyl acetals by NCINA
[C6H5CH(OC4H9)2] = 5.0 x 10-2 M [NCINA] = 5.0 x 10-3 M
[NaClO4] = 1.0 x 10-1 M CH3CN = 100%
| Compound |
k1 x 10-4 (s-1) |
Ea kJ mol-1 |
∆H# kJ mol-1 |
∆S# j mol-1 K-1 |
∆G# kJ mol-1 |
||||
|
313 K |
318 K |
323 K |
328 K |
333 K |
|||||
| R = ethyl |
4.02 |
5.94 |
8.14 |
12.03 |
16.15 |
60.46 |
57.77 |
54.65 |
75.42 |
| R = propyl |
4.23 |
6.12 |
8.19 |
12.24 |
16.22 |
58.61 |
55.93 |
57.11 |
74.37 |
| R = butyl |
4.35 |
6.37 |
8.29 |
12.35 |
16.37 |
57.43 |
57.75 |
58.64 |
73.69 |
| R = iso-butyl |
4.51 |
6.42 |
8.32 |
12.58 |
16.51 |
56.64 |
53.95 |
59.71 |
73.24 |
| R = iso-amyl |
4.62 |
6.57 |
8.37 |
12.89 |
16.84 |
56.443 |
53.757 |
59.95 |
73.12 |
| R = benzyl |
4.83 |
6.89 |
8.46 |
13.11 |
17.07 |
54.89 |
52.21 |
61.99 |
72.23 |
Conclusion
In summary a new and attractive method has been adopted for the preparation of N-chloroisonipecotamide in 90% yield in 1h. The reaction conditions employed and the isolated yield obtained seems to be considerably better than those reported in the literature. Further more this method permits the N-chlorination of cyclic compounds such as isonipecotamide in high yield. Also the oxidation potential of the oxidant is studied by the oxidation of series of benzaldehyde di-n-alkyl acetals by NCINA.
References
- Dhum P. S., Mobe N. U. and Salunkhe M. M., Synth. Commun., 2001, 31, 3653. Caribano V., Rodriguez J. F., Santose M., Sanz-Tejedor M. A., Carreno M.C., Gonzaloz G. and Garcia-Ruano J.L., Synthesis, 14, 2175(2001).
- Thenraja D. S., Subramaniam P. and Srinivasan C., J .Chem. Soc .Perkin Trans., 2, 2125(2002). Mukaiyama T., Mastsuo J. I., Lida D. and Kitagawa H., Chem. Lett., 8, 846(2001).
- Bharat Singh, Singh A. K., Singh N. B., and Saxena B. B. L., Tetrahedran, 40(24), 5203(1984).Cantor S. E., Adhes age., 17, 17(1974); Chem abstr., 81, 122012y(1974).
- Farook Mohamed N. A., J. Iranian Chem. Soc., 3(4), 378(2006).
- Iranpoor N., Firouzabadi H. and Shaterian H. R., Tetrahedron Lett., 44, 4769(2003).
- Mathiyalagan N., J. Indian Chem. Soc., 88(6), 865(2011).
- Ramakrishnan P. S., Asian J. Chem., 12(4), 1096(2000).
- Ramakrishnan P. S. and Nambi K., J. Indian Chem. Soc., 77(5), 232(2000).
- Basheer Ahamed K. A., Sheik Dawood S., Baskaran P. and Nambi K., J. Indian Chem. Soc., 73(12), 687(1996).
- Chockalingam P., Ramakrishnan P. S., Arulraj S. J. and Nambi K., J. Indian Chem. Soc., 69(5), 247(1992).
- Nambi K., Ahamed K. A. Basheer and Arulraj S. J., J. Indian Chem. Soc., 65(2), 85(1988).
- Basheer Ahamed K. A., Nambi K. and Arulraj S. J., Indian J. Chem., 26A (8), 672(1987).
- Singh, Ajaya Kumar Negi, Reena Jain, Bhawana Katre, Yokraj Singh, Surya Prakash Sharma and Virender K., Indus. Eng. Chem. Res., 50(14), 8407(2011).
- Ulagi Ratinum, Kuselan Perumal and Karunakaran Chockalingam, Monatshefte fuer. Chemie., 132(7), 799(2001).
- Priya V., Balasubramaniyan M. and Mathiyalagan N., J. Chem. Pharm. Res., 3(1), 522(2011).
- Mohamed Farook N. A. and Seyed Dameem G. A., E. J. Chem., 8(2), 479(2011).
- Pushpalatha L., Afinidad, 68, 57(2011).
- Connell R. D., In Comprehensive Organic Functional Group Transformations, Vol V, Moody CJ, Pregamon Press, 309(1995).
- Curini M., Epifano F., Marcotullio M. C., Rosati O. and Tsadjout A., Synlett, 23, 2423(2000): Back T. G., lai E. K. Y. and Morzycki J. W., Heterocycles, 32, 481(1991).
- Gabriela Fonseca Mendonca, Ramos Magalhaes, Maricio CS De Mattos and Pierre M Esteves, J. Braz. Chem. Soc., 16(4), 695(2005).
- Gene A Heigel, Tyrone J Hogenauer and Justin C Lewis, Synth. Commun., 35, 2099(2005).
- Nair G. R., Lalithakumari R. and Senan P. L., Talanta, 25(9), 525(1978).
- Priya V. and Mathiyalagan N., Asian J. Chem., 23(4), 1871(2011).
- Pushpalatha L. and Vivekanandan K., J. Indian Chem. Soc., 84, 1119(2007).
- Balasubramaniyan M. and Mathiyalagan N., Orient. J. Chem., 26(4), 1541(2010).
- Vivekanandan K. and Nambi K., Indian J. Chem., 35(B), 1117(1996).
- Coetzee J. F., Cunnigham G. P., Mc Guire D. K. and Padmanabhan G. R., Anal. Chem., 34, 1139(1962).
- Deno N. C. and Neil H Potter, J. Am. Chem. Soc., 89, 3550(1967).
- Gopalakrishnan G. P., Pai R. and Venkatasubramanian N., Indian J. Chem., 18, 92(1979).
- Shenbagam K. and Mathiyalagan N., J. Indian Chem. Soc., 89, 1343(2012).
- Amis E. S., J. Chem. Educ., 30, 351(1953).over [oxidant]. Mixture containing requisite amounts of solutions of acetal, sodium perchlorate and

This work is licensed under a Creative Commons Attribution 4.0 International License.









