Synthesis, Characterization and Pharmacological Studies of Some Substituted Fluoroquinolones
Faiz Mohd. Abdullah* and Arvind Kumar Singh
Kamla Nehru Institute of Management and Technology, (Faculty of Pharmacy), Faridipur, Sultanpur - 228 118, India.
Corresponding Author E-mail: fmabd2006@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/290231
Certain N-1 substituted fluoroquinolonic derivatives were synthesized and evaluated for antimicrobial and antioxidant activities. N-1 alkyl/ aryl/ aryl sulphonyl substituted derivatives of the title compounds have been synthesized to identify newer fluoroquinolones which have better efficacy, lesser side effects and well tolerability. The biological evaluation of the synthesized fluoroquinolone derivatives was carried out using agar-well diffusion method and compounds FI-ETH, FII-SUL, FIII-ATY, FIV-BZO, and FV-BZY were found to be active against both Gram-positive and Gram-negative bacteria having activity comparable to that of standard drug i.e. Ofloxacin 10mg/ml. N-1 substituted moiety is mostly active against strain is S. aureus, K. Pneumonia, and E. coli with concentration of 100 - 150μg/ml, and less active against strain is B. subtilis. And secondly, antioxidant activity is that of show the better activity by this four method DPPH Free Radical Scavenging Assay, Hydrogen Peroxide Radical Scavenging Activity, Nitric Oxide Assay, and Reducing Power Assay. All the synthesized compounds show the better antioxidant activity and in fourth one method result is mostly capable reducing power activity of the synthesized compounds.
KEYWORDS:Fluoroquinolones; Antibacterials; Antioxidant agent
Download this article as:| Copy the following to cite this article: Abdullah F. M, Singh A. K. Synthesis, Characterization and Pharmacological Studies of Some Substituted Fluoroquinolones. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Abdullah F. M, Singh A. K. Synthesis, Characterization and Pharmacological Studies of Some Substituted Fluoroquinolones. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22180 |
Introduction
The first clinically useful quinolone was naldixic acid, discovered by Lesher and co-workers in 1962, which was generated from chloroquine, an antimalarial agent (1). It was active against some Gram negative bacteria and had limited usefulness because of its high protein binding (approximately 90%) and little half life (about 1.5 h) (6). Unfortunately, bacteria could develop a rapid resistance to this agent (7, 11). Flumequine was the first fluoroquinolone which was patented in 1973, after that many fluoroquinolones have been patented and are still used today, including norfloxacin (1978), pefloxacin (1979), enoxacin (1980), fleroxacin (1981), ciprofloxacin (1981) and ofloxacin (1982) (1). An advantage of these compounds over previous ones is their broad spectrum. A big revolution was made in 1980 ís when an analog of naldixic acid, enoxacin was derived with significantly increased spectrum of activity against Gram negative or Gram positive bacteria (8). An antioxidant is a molecule capable of inhibiting or preventing the oxidation of other molecules. Antioxidants are substances that may protect cells from the damaging effects of oxygen radicals, highly reactive chemicals that play a part in atherosclerosis, some forms of cancer and reperfusion injuries (4). Free radicals are created when cells use oxygen to generate energy. These by-products are generally reactive oxygen species (ROS) such as super oxide anion, hydroxyl radical and hydrogen peroxide that result from the cellular redox process. At low or moderate concentrations, (ROS) exert beneficial effects on cellular responses and immune function but at high levels, free radicals and oxidants generate oxidative stress, a deleterious process that can damage cell structures, including lipids, proteins, and DNA (9). Oxidative stress plays a major part in the development of chronic and degenerative ailments such as cancer, autoimmune disorders, rheumatoid arthritis, cataract, aging, cardiovascular and neurodegenerative diseases, (14, 9). The human body has several mechanisms to counteract oxidative stress by producing antioxidants, which are either naturally produced in situ, or externally supplied through foods and/or supplements. These antioxidants act as free radical scavengers by preventing and repairing damages caused by ROS, and therefore can enhance the immune defense and lower the risk of cancer and degenerative diseases (9). In recent years, there is an increasing interest in finding antioxidant phytochemicals, because they can inhibit the propagation of free radical reactions, protect the human body from diseases (12).
Methodology
Synthesis of derivative FI-ETH Equimolar amounts of 3-chloro-4-fluoroaniline (i), and diethylethoxymethylene malonate (ii) were condensed at 1450C to get 3-chloro-4-fluoroanilinomethylene malonic diethyl ester (iii), which was then cyclized by heating at 2500C in diphenyl ether to get ethyl 7-chloro-6-fluoro-4-oxo1, 4-dihydroquinoloine-3-carboxylate (iv). Whole reaction mixture change into the semisolid mass having white to pale yellow appearance and washed with acetone to get almost white solid recrystillised using DMF as solvent. Showed in scheme 1 fig. I.
Synthesized derivatives (FII-SUL, FIII-ATY, FIV-BZO, and FV-BZY) are synthesized by this following method. ethyl 7-chloro-6-fluoro-4-oxo1, 4-dihydroquinoloine-3-carboxylate (iv) 0.01 mol was added to 10 ml. of DMF, followed by addition of N-1 position (R) (0.01 mol). The reaction mixture was heated to dissolve the 3-acid which was partially soluble in cold condition, and then anhydrous potassium carbonate (0.02 mol) was added to the reaction mixture. The whole reaction mixture was heated to 1200 – 1400C and stirred for 5-8 hrs. then, the reaction mixture was poured onto the crushed ice or ice cold water, washed with cold water to remove DMF and potassium carbonate if any, the solid (v) obtained was recrystillised from acetone to get the derivative. Showed in scheme 2 fig. II. All synthesized derivatives checked by TLC technique using adsorbent silica gel G of Merck specialties Pvt. Ltd., Mumbai and each derivatives are isolate & purified by column chromatography using silica gel mesh size 60-120 of Merck specialties Pvt. Ltd., Mumbai.
In-vitro antibacterial activity (5)
Agar- Well Diffusion Method: Petriplates containing 20 ml Muller Hinton medium were seeded with 24 hr culture of bacterial strains. Wells were cut and 20 μl of the given sample (of different concentrations) were added. The plates were then incubated at 37°C for 24 hours. The antibacterial activity was assayed by measuring the diameter of the inhibition zone formed around the well. Ofloxacin were used as a positive control.
In-vitro antioxidant activity by
DPPH Free Radical Scavenging Assay (2, 10)
The stable 1,1-diphenyl-2-picryl hydrazyl radical (DPPH) was used for determination of free radical-scavenging activity of the sample Different concentrations (50 – 250 µg/ml) of each sample were added, at an equal volume, to 90% methanolic solution of DPPH (100 μM). After 15 min at room temperature, the absorbance was recorded at 517 nm. The experiment was repeated for three times. Ascorbic acid were used as standard controls. IC50 values denote the concentration of sample, which is required to scavenge 50% of DPPH free radicals.
Hydrogen Peroxide Radical Scavenging Activity (4)
The ability of the sample (FI-ETH, FII-SUL, FIII-ATY, FIV-BZO, and FV-BZY) to scavenge hydrogen peroxide was determined. A solution of hydrogen peroxide (40 mM) was prepared in phosphate buffer (pH 7.4). The concentration of hydrogen peroxide was determined by absorption at 230 nm using a spectrophotometer. Sample (50 – 250 µg/ml) in distilled water, and 1 ml added to 2.4 ml. of 0.1 M phosphate buffer solution and a hydrogen peroxide solution (600 µl, 40 mM). The absorbance of hydrogen peroxide at 230 nm was determined after 10 minutes against a blank solution containing phosphate buffer without hydrogen peroxide. The percentage of hydrogen peroxide scavenging by the extracts and standard compounds was calculated as follows: % Scavenged [H2O2] = [(Ao − A1)/Ao] × 100 where Ao was the absorbance of the control and A1 was the absorbance in the presence of the sample and standard.
Nitric Oxide Assay (13)
The procedure is based on the principle that, sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide which interacts with oxygen to produce nitrite ions that can be estimated using Griess reagent. Scavengers of nitric oxide compete with oxygen, leading to reduced production of nitrite ions. For the experiment, sodium nitroprusside (5 mM), in phosphate-buffered saline, was mixed with different concentrations (50 – 250 µg/ml) of each sample dissolved in methanol and incubated at room temperature for 30 min. After the incubation period, 1.5 ml incubated solution was removed and diluted with 1.5 ml of Griess reagent (1% sulphanilamide, 2% phosphoric acid and 0.1% N-1-naphthyethylenediamine dihydrochloride) was added. The absorbance of the chromophore formed was read at 546 nm. Rutin was used as standard.
Reducing Power Assay (3)
The reducing power assay of sample was determined. Different amounts of each sample (50 -800 μg/ ml) in 1 ml of methanol were mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferrocyanide [K3Fe(CN)6] (2.5 ml, 1%). The mixture was incubated at 50oC for 20 min. A portion (2.5 ml) of trichloroacetic acid (10%) was added to the mixture to stop the reaction, which was then centrifuged at 3000 rpm for 10 min. The upper layer of solution (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3
(0.5 ml, 0.1%), and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. Ascorbic acid used as a standard.
Spectral Analysis
All synthesized derivatives done characterized by 1H NMR, MASS, and FT-IR.
FI-ETH: FT-IR (cm-1)
1445 (C-N stretch of quinolines having C=C-N), 1044 (C-F), 751 (C-Cl), 1650 (C=C-C=O), 1733 (C=C-COOR), 1618 (N-H bending).
MS (m/z)
269 (M+) C12H9ClFNO3 calculated value: C= 53.53, H= 3.35, Cl= 13.20, F= 7.05, N= 5.2, O= 17.84; 270 (M+) Found value: C= 53.33, H= 3.33, Cl= 13.14, F= 7.03, N= 5.18, O= 17.77.
+H NMR (dmso d6 300 MHz) δ ppm= 7.06 (1H, s, J= 7.021), 7.46 (1H, s, J= 7.424), 4.15 (1H, s, J= 4.133), 1.13 (3H, m, J= 1.056), 4.4 (2H, m, J= 4.353), 8.0 (1H, s, J= 7.954).
FII-SUL: FT-IR (cm-1)
1450 (C-N stretch of quinolines C=C-N), 1044 (C-F), 637 (C-Cl), 1635 (C=C-C=O), 1733 (C=C-COOR), 3325 (aromatic C-H), 2972 (C-H alkane), 1380 (SO2 Symmetric), 1274 (SO2 Asymmetric).
MS (m/z)
423 (M+), C19H15ClFNO5S calculated value: C= 53.90, H= 3.54, Cl= 8.39, F= 4.49, N= 3.30, O= 18.91, S= 7.5; 422 (M+) Found value: C= 53.77, H= 3.53, Cl= 8.37, F= 4.48, N= 3.30, O= 18.86, S= 7.56.
+H NMR (dmso d6 300 MHz) δ ppm= 7.06 (1H, s, J= 7.021), 7.46 (1H, s, J= 7.424), 1.13 (3H, m, J= 1.056), 4.4 (2H, m, J= 4.353), 8.0 (1H, s, J= 7.954), 7.3 (2H, m, J= 7.414), 7.71 (2H, m, J= 7.734), 2.28 (3H, m, J= 2.281).
FIII-ATY: FT-IR (cm-1)
1425 (C-N stretch of quinolines C=C-N), 1044 (C-F), 659 (C-Cl), 1649 (C=C-C=O), 1733 (C=C-COOR), 2973 (C-H alkane).
MS (m/z)
311 (M+), C14H11ClFNO4 calculated value: C= 54.01, H= 3.5, Cl= 11.41, F= 6.1, N= 4.5, O= 20.5; 311 (M+) Found value: C= 54.01, H= 3.5, Cl= 11.41, F= 6.1, N= 4.5, O= 20.5.
+H NMR (dmso d6 300 MHz) δ ppm= 7.83 (1H, m, J= 7.844), 7.54 (1H, m, J= 7.615), 8.75 (1H, s, J= 8.728), 1.13 (3H, m, J= 1.056), 4.4 (2H, m, J= 4.353), 2.27 (3H, s, J= 2.299).
FIV-BZO: FT-IR (cm-1)
1466 (C-N stretch of quinolines C=C-N), 1030 (C-F), 618 (C-Cl), 1690 (C=C-C=O), 3099 (aromatic C-H).
MS (m/z)
373 (M+) C19H13ClFNO3 calculated value: C= 61.12, H= 3.48, Cl= 9.51, F= 5.09, N= 3.75, O= 17.15; 373 (M+) Found value: C= 61.12, H= 3.48, Cl= 9.51, F= 5.09, N= 3.75, O= 17.15.
+H NMR (dmso d6 300 MHz) δ ppm= 7.83 (1H, m, J= 7.844), 7.54 (1H, m, J= 7.615), 8.75 (1H, s, J= 8.728), 1.13 (3H, m, J= 1.056), 4.4 (2H, m, J= 4.353), 1.13 (3H, m, J= 1.056), 4.4 (2H, m, J= 4.353), 7.79 (2H, m, J= 7.812), 7.34 (2H, m, J= 7.386), 7.57 (1H, s, J= 7.601).
FV-BZY: FT-IR (cm-1)
1494 (C-N stretch quinolines having C=C-N), 881 (C-F), 658 (C-Cl), 1655 (C=C-C=O), 3423 (Aromatic C-H), 2969 (C-H alkane).
MS (m/z)
359 (M+) C19H15ClFNO3 calculated value: C= 63.50, H= 4.17, Cl= 9.88, F= 5.29, N= 3.89, O= 13.37; 359 (M+) Found value: C= 63.50, H= 4.17, Cl= 9.88, F= 5.29, N= 3.89, O= 13.37.
+H NMR (dmso d6 300 MHz) δ ppm= 7.23 (1H, s, J= 7.299), 7.97 (1H, m, J= 7.973), 4.26 (2H, m, J= 4.253).
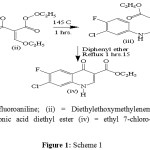 |
Figure 1: Scheme 1: Click here to View figure |
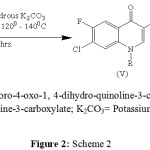 |
Figure 2: Scheme 2: Click here to View figure |
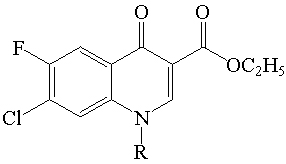
Table 1: Physico-chemical properties of the Derivatives
|
Derivatives |
Substituents R |
Melting Point |
Yield (%) |
Rf |
Solvent System |
|
FI-ETH |
H |
2500-2530C |
80 |
0.64 |
Ethyl Acetate:Benzene:Acetone 5:2:2
|
|
FII-SUL |
C7H7ClO2S |
2610-2660C |
70 |
0.9 |
Ethyl Acetate:Benzene:Acetone 4:2:2
|
|
FIII-ATY |
C2H3ClO |
2430-2480C |
67 |
0.82 |
Ethyl Acetate:Benzene:Acetone 5:2.5:2
|
|
FIV-BZO |
C7H5ClO |
2560-2600C |
65 |
0.64 |
Ethyl Acetate:Chloroform:Acetone 5:2:2
|
|
FV-BZY |
C7H7Cl |
2570-2610C |
62 |
0.48 |
Ethyl Acetate:Hexane 4:1
|
Results and Discussion
Chemistry
The preparation of N-1 substituted fluoroquinolone derivative I (ethyl 7-chloro-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate) is outlined in scheme 1. 3-chloro-4-fluoroaniline (i) was treated with diethylethoxymethylenemalonate (ii) with 1450C heat for 1 hrs. approximate and then treated with diphenyl ether heated at 2500C approximate and gives its ethyl 7-chloro-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (iv) over all yield 80%. The synthetic pathways for preparation of N-1 substitution in (iv) by treated with Anhydrous K2CO3, DMF at 1200- 1400C for 5 – 8 hrs. N-1= R (P-toluenesulphonyl chloride, Acetyl chloride, Benzoyl chloride, Benzyl chloride respectively.) is outlined in scheme 2. The physicochemical parameters are recorded are given in the table 2.
In-vitro antibacterial activity All synthesized derivatives are active against gram (+ve) and (-ve) bacterial growth but increasing concentration of the derivatives that is increased inhibitory action on bacterial growth. This inhibition seen on B. subtilis, S. aureus, and E. coli with concentration of 150 µg/ml. as shows in table 2.
In-vitro antioxidant activity
Antioxidant activity shown is excellent in all synthesized derivatives, and antioxidant activity done by four methods DPPH free radical scavenging assay is capable of hydrogen donating nature and synthesized all derivatives show the hydrogen donating nature, Hydrogen peroxide radical scavenging activity ability to, nitric oxide assay is Outlined in table 3. and reducing power assay is showed the increasing the absorbance of reaction mixture to increase the reducing power, and all synthesized derivatives show the reducing power. outlined in table 4.
Table 2: Test Result of Derivatives with Standard Drug Ofloxacin
|
Organism |
Zone of Inhibition (mm) |
|||||||||||||||
|
Derivatives with concentration (µg/ml) |
||||||||||||||||
|
FI-ETH |
FII-SUL |
FIII-ATY |
FIV-BZO |
FV-BZY |
Ofloxacin (10mg/ml) |
|||||||||||
|
50 |
100 |
150 |
50 |
100 |
150 |
50 |
100 |
150 |
50 |
100 |
150 |
50 |
100 |
150 |
||
| K. Pneumonia |
15 |
21 |
28 |
14 |
22 |
30 |
19 |
24 |
31 |
15 |
19 |
21 |
17 |
19 |
22 |
38 |
| B. subtilis |
26 |
34 |
39 |
25 |
35 |
40 |
23 |
36 |
33 |
20 |
23 |
32 |
29 |
32 |
34 |
42 |
| S. aureus |
22 |
24 |
40 |
20 |
23 |
36 |
29 |
32 |
38 |
22 |
25 |
35 |
23 |
25 |
27 |
41 |
| E. coli |
29 |
30 |
32 |
28 |
29 |
31 |
25 |
30 |
32 |
24 |
29 |
30 |
23 |
26 |
30 |
34 |
Table 3: Antioxidant activity Test Result of Derivatives
|
Derivatives |
DPPH radical scavenging, IC50 (μg/ ml)a |
H2O2 scavenging IC50 (μg/ ml)b |
NO-scavenging IC50 (μg/ ml)c |
|
FI-ETH |
81.69 |
287.71 |
79.24 |
|
FII-SUL |
14.98 |
143.51 |
61.73 |
|
FIII-ATY |
68.63 |
225.76 |
31.94 |
|
FIV-BZO |
80.32 |
245.18 |
68.34 |
|
FV-BZY |
92.9 |
286 |
83.46667 |
Where a IC 50 for Ascorbic acid (standard drug) is 32.0 (µg/ml); b IC 50 for Ascorbic acid (standard drug) is 67.36 (µg/ml); c IC 50 for Rutin (standard drug) is 15.07 (µg/ml).
Table 4: Antioxidant activity (Reducing Power Assay) of Derivatives
|
Concentration (µg/ml) |
Ascorbic acid (std. drug) Absorbance |
FI-ETH Absorbance |
FII-SUL Absorbance |
FIII-ATY Absorbance |
FIV-BZO Absorbance |
FV-BZY Absorbance |
|
50 |
0.029 |
0.061 |
0.092 |
0.074 |
0.080 |
0.084 |
|
100 |
0.036 |
0.070 |
0.101 |
0.083 |
0.089 |
0.093 |
|
150 |
0.057 |
0.098 |
0.129 |
0.092 |
0.106 |
0.121 |
|
200 |
0.078 |
0.112 |
0.140 |
0.116 |
0.130 |
0.145 |
|
250 |
0.105 |
0.129 |
0.167 |
0.133 |
0.147 |
0.162 |
Acknowledgment
I special thank to Dr. B. Kundu Deputy Director & Head of SAIF CDRI Lucknow (U.P.).
References
- Appelbaum P.C., Hunter P.A.: Int. J. Antimicrob. Agents 16, 5 (2000).
- Ara N. and Nur H., In vitro antioxidant activity of methanolic leaves and flowers extract of Lippia alba, Research Journal of Medicine and Medical Sciences, 2009, 4, 107-110.
- Arulpriya P., Lalitha P. and Hemalatha S., Invitro antioxidant testing of the extracts of Samanea saman (Jacq.) Merr, Der Chemi
- Chanda S. and Dave R., In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview, African Journal of Microbiology Research, 2009, 3, 981-996.
- Cleidson Valgas; Simone Machado de Souza; Elza F A Smânia; Artur Smânia Jr., screening methods to determine antibacterial activity of natural products; Brazilian Journal of Microbiology, 38 (2007): 369-380.
- Dollery C.: Norfloxacin, in Therapeutic Drugs, Churchill Livingstone, Harcourt Brace and Co. Ltd., London 1999, p. N137-N140.
- Pandey S. N.: Antimicrobial Agents-Sulphonamides and Quinolones, in A Text Book of Medicinal Chemistry (Synthetic and Biochemial Approach) Mahavir Press, Bhelpur, S. G. Publisher, Varanasi, 2003, , p. 547-585,.
- Patrick G. L.: Antibacterial agents, in An Introduction to Medicinal Chemistry, Oxford University Press, Oxford, New York 2003, p. 379-435.
- Pham-Huy LA, He H and Pham-Huyc C (2008). Free Radicals, Antioxidants in Disease and Health. International Journal of Biomedical Science, 4 (2): 89-96.
- Rakesh S. U., Patil P. R. and Salunkhe V.R., Free radical scavenging activity of hydroalcoholic extracts of dried flowers of Nymphaea stellata Wild., International Journal of Pharma and Bio Science, 2010, 1, 4-9.
- Sarkozy G.: Vet. Med. Czech. 46, 257 (2001).
- Terao J and Piskula MK Flavonoids as inhibitors of lipid peroxidation in membranes. In Rice-Evans CA and Packer L. (editor), Flavonoids in health and disease. Marcel Dekker. New York, (1997), pp.277-295.
- Umamaheswari M. and Chatterjee T.K., In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract, African Journal of Traditional, Complementary and Alternative Medicine, 2008, 5, 61-73.
- Willcox J. K., Ash S. L. and Catignani G. L. Antioxidants and prevention of chronic disease.Critical Reviews in Food Science and Nutrition, 44, 2004: 275-295.

This work is licensed under a Creative Commons Attribution 4.0 International License.









