Synthesis and study of antibacterial activity of some 1-phenyl-3-aryl-5-(4-(2-ethanoloxy) phenyl)-1H-pyrazoles
Anju Goyal1, Neelam Jain2 and Sandeep Jain3
1Chitkara College of Pharmacy, Rajpura, Patiala-140 401, India.
2Department of Pharmaceutical Education and Research, Bhagat Phool Singh Mahila Vishwavidyalaya, Khanpur Kalan, Sonipat - 131305, India.
3Drug Discovery and Research Laboratory, Department of Pharmaceutical Sciences, Guru Jambheshwar University of Science and Technology, Hisar - 125 001, India.
Corresponding Author E-mail: drsjain1969@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/290223
A series of 1-phenyl-3-aryl-5-(4-(2-ethanoloxy) phenyl)-1H-pyrazoles were synthesized from chalcones and studied for their in vitro antibacterial activity. Chalcones i.e.,1-aryl-3-(4-hydroxyphenyl) prop-2-en-1-ones, 1 on reaction with phenyl hydrazine yielded the corresponding 1-phenyl-3-aryl-5-(4-hydroxyphenyl)-1H-pyrazoles 2 which on further reaction with 3-chloroethanol furnished the title compounds 3. These compounds were characterized by CHN analyses, IR, mass and 1H NMR spectral data. All the compounds were evaluated for their in vitro antibacterial activity against two Gram negative strains (Escherichia coli and Pseudomonas aeruginosa) and two Gram positive strains (Bacillus subtilis and Staphylococcus aureus) and their minimum inhibitory concentration (MIC) were determined.
KEYWORDS:Chalcones; 1H-pyrazoles; antibacterial activity; Minimum inhibitory concentration (MIC)
Download this article as:| Copy the following to cite this article: Goyal A, Jain N, Jain S. Synthesis and study of antibacterial activity of some 1-phenyl-3-aryl-5-(4-(2-ethanoloxy) phenyl)-1H-pyrazoles. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Goyal A, Jain N, Jain S. Synthesis and study of antibacterial activity of some 1-phenyl-3-aryl-5-(4-(2-ethanoloxy) phenyl)-1H-pyrazoles. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22157 |
Introduction
The wide-spread exploitation of chemotherapeutic agents for the treatment of infectious diseases leads to the development of microbial resistance to existing drugs. The emergence of resistance to the major classes of antibacterial drugs is recognized as a serious health concern. The hunt for novel antibacterial agents with different mode of actions will always remain an important and challenging task1. Compounds containing heterocyclic ring systems continue to attract considerable interest due to their wide range of biological activities. Amongst them five membered heterocyclic compounds occupy a unique place in the realm of natural and synthetic organic chemistry. Five membered heterocycles like pyrazoles have been found to exhibit wide application as pharmaceutical agents. In recent years, attention has increasingly been given to the synthesis of pyrazole derivatives as a source of new antibacterial agents. Pyrazole derivatives have been reported to possess diverse biological activities such as antibacterial2,3, antifungal4,5, herbicidal6, insecticidal7, anti-inflammatory8,9, anticonvulsant10, antitumor11, anti-oxidant12, etc. Further, phenoxyethanol has been used as antimicrobial preservative in vaccine preparations13. These reports including our earlier work on the synthesis and antibacterial evaluation of pyrazoles having phenoxy alkanol functionality14-16 inspired us to undertake the synthesis of some more 1H-pyrazoles bearing phenoxy ethanol moiety. The synthesized compounds were characterized on the basis of elemental analysis, IR, 1H NMR and Mass spectral data. All the compounds were screened for their in vitro antibacterial activity against two Gram negative strains (Escherichia coli and Pseudomonas aeruginosa) and two Gram positive strains (Bacillus subtilis and Staphylococcus aureus) respectively.
Experimental
Chemistry
The purity of all the synthesized compounds was checked by thin-layer chromatography on silica gel G as a stationary phase and different solvent systems as a mobile phase using iodine vapors as detecting agent. Melting points were determined by the Tempo melting point determination apparatus in open capillary tubes and are uncorrected. Elemental analyses were carried out on Perkin Elmer 2400 CHN Elemental Analyser. Infrared spectra were recorded on Shimadzu 8000 FTIR Spectrophotometer in KBr phase. Proton NMR spectra were done on Bruker Avance II 400 NMR Spectrometer using tetra-methyl silane as internal standard. Mass spectra of the compounds were carried out on Waters Micromass Q-Tof Micro Mass Spectrometer using electrospray ionization (ESI) technique. Chalcones 1a–g were synthesized by a base-catalyzed Claisen-Schmidt condensation reaction of appropriately substituted acetophenones and 4-hydroxy benzaldehyde17 and 1-phenyl-3-aryl-5-(4-hydroxyphenyl)-1H-pyrazoles 2a–g were prepared from the chalcones 1a–g following the procedure described in the literature18.
General procedure for the synthesis of 1-phenyl-3-aryl-5-(4-(alkanoloxy) phenyl) 1H-pyrazoles
1-Phenyl-3-aryl-5-(4-hydroxyphenyl)-1H-pyrazoles (2a-g, 0.01 M) and 2-chloroethanol (0.01 M) were refluxed in acetone (50 ml) in presence of triethylamine (0.01 M) for about four hours. Excess of solvent was removed under reduced pressure. The residue thus obtained was washed thoroughly with cold distilled water, dried and then re-crystallized from ethanol. The physical and analytical data of the synthesized title compounds (3a-g) are given as follows.
1, 3-Diphenyl-5-(4-(2-ethanoloxy phenyl)-1H-pyrazole
Yield: 78%; m.p.: 70-72oC; IR (KBr, cm–1): 3344 (O–H), 3065 (aromatic C–H str), 2916 (C–H), 1465, (CH2), 1255 (C–O–C), 1065 (C–O), 832, 730 & 690 (aromatic C–H def); 1HNMR (CDCl3): δ (ppm) 8.05-7.09 (m, 14H, ArH), 7.02 (s, 1H, =CH–), 4.33-4.31 (t, 2H, HO– CH2–CH2–O–Ar), 3.59-3.57 (t, 2H, HO–CH2–CH2–O–Ar), 3.65 (s, 1H, O–H); MS, m/z (%): 357 [M+H]+ (100%). Anal.: Calcd. for C23H20N2O2: C, 77.51; H, 5.66; N, 7.86. Found: C, 77.58; H, 5.60; N, 7.81.
1-Phenyl-3-(4-methylphenyl)-5-(4-(2-ethanoloxy) phenyl)-1H-pyrazole
Yield: 73%; m.p.: 81-83oC; IR (KBr, cm–1): 3340 (O–H), 3063 (aromatic C–H str), 2918 (C–H), 1467, (CH2), 1255 (C–O–C), 1065 (C–O), 832, 735 & 692 (aromatic C–H def); 1HNMR (CDCl3): δ (ppm) 7.67-7.08 (m, 13H, ArH), 7.02 (s, 1H, =CH–), 4.32-4.30 (t, 2H, HO–CH2–CH2–O–Ar), 3.58-3.56 (t, 2H, HO–CH2–CH2–O–Ar), 3.65 (s, 1H, O–H), 2.34 (s, 3H, CH3–Ar); MS, m/z (%): 371 [M+H]+ (100%). Anal.: Calcd. for C24H22N2O2: C, 77.81; H, 5.99; N, 7.56. Found: C, 77.89; H, 5.90; N, 7.51.
1-Phenyl-3-(4-methoxyphenyl)-5-(4-(2-ethanoloxy) phenyl)-1H-pyrazole
Yield: 69%; m.p.: 87-89oC; IR (KBr, cm–1): 3342 (O–H), 3060 (aromatic C–H str), 2916 (C–H), 1463, (CH2), 1254 (C–O–C), 1066 (C–O), 830, 730 & 692 (aromatic C–H def); 1HNMR (CDCl3): δ (ppm) 7.62-7.08 (m, 13H, ArH), 7.01 (s, 1H, =CH–), 4.33-4.31 (t, 2H, HO–CH2–CH2–O–Ar), 3.83 (s, 3H, CH3O–Ar), 3.59-3.57 (t, 2H, HO–CH2–CH2–O–Ar), 3.65 (s, 1H, O–H); MS, m/z (%): 387 [M+H]+ (100%). Anal.: Calcd. For C24H22N2O3: C, 74.59; H, 5.74; N, 7.25. Found: C, 74.51; H, 5.80, N, 7.30.
1-Phenyl-3-(4-chlorophenyl)-5-(4-(2-ethanoloxy) phenyl)-1H-pyrazole
Yield: 82%; m.p.: 68-70oC; IR (KBr, cm–1): 3340 (O–H), 3069 (aromatic C–H str), 2919 (C–H), 1461, (CH2), 1253 (C–O–C), 1066 (C–O), 833, 733 & 690 (aromatic C–H def); 1HNMR (CDCl3): δ (ppm) 8.01-7.08 (m, 13H, ArH), 7.02 (s, 1H, =CH–), 4.32-4.30 (t, 2H, HO–CH2–CH2–O–Ar), 3.56-3.54 (t, 2H, HO–CH2–CH2–O–Ar), 3.65 (s, 1H, O–H); MS, m/z (%): 391 [M+H]+ (100%), 393 [M+2+H]+ (35%). Anal.: Calcd. For C23H19ClN2O2: C, 70.68; H, 4.90; N, 7.17. Found: C, 70.60; H, 4.96; N, 7.11.
1-Phenyl-3-(4-bromophenyl)-5-(4-(2-ethanoloxy) phenyl)-1H-pyrazole
Yield: 79%; m.p.: 70-71oC; IR (KBr, cm–1): 3338 (O–H), 3065 (aromatic C–H str), 2916 (C–H), 1465, (CH2), 1255 (C–O–C), 1065 (C–O), 832, 730 & 690 (aromatic C–H def); 1HNMR (CDCl3): δ (ppm) 7.78-7.08 (m, 13H, ArH), 7.02 (s, 1H, =CH–), 4.32-4.30 (t, 2H, HO–CH2–CH2–O–Ar), 3.56-3.54 (t, 2H, HO–CH2–CH2–O–Ar), 3.65 (s, 1H, O–H); MS, m/z (%): 435 [M+H]+ (100%), 437 [M+2+H]+ (98%). Anal.: Calcd. For C23H19BrN2O2: C, 63.46; H, 4.40; N, 6.44. Found: C, 63.40; H, 4.47; N, 4.41.
1-Phenyl-3-(4-fluorophenyl)-5-(4-(2-ethanoloxy) phenyl)-1H-pyrazole
Yield: 66%; m.p.: 83-84oC; IR (KBr, cm–1): 3342 (O–H), 3068 (aromatic C–H str), 2917 (C–H), 1465, (CH2), 1255 (C–O–C), 1065 (C–O), 832, 730 & 692 (aromatic C–H def); 1HNMR (CDCl3): δ (ppm) 8.15-7.07 (m, 13H, ArH), 7.01 (s, 1H, =CH–), 4.33-4.31 (t, 2H, HO–CH2–CH2–O–Ar), 3.57-3.55 (t, 2H, HO–CH2–CH2–O–Ar), 3.65 (s, 1H, O–H); MS, m/z (%): 375 [M+H]+ (100%). Anal.: Calcd. for C23H19FN2O2: C, 73.78; H, 5.11; 5.07; N, 7.48. Found: C, 73.71; H, 5.16; N, 7.42.
1-Phenyl-3-(4-nitrophenyl)-5-(4-(2-ethanoloxy) phenyl) 1H-pyrazole
Yield: 65%; m.p.: 59-61oC; IR (KBr, cm–1): 3340 (O–H), 3060 (aromatic C–H str), 2916 (C–H), 1465, (CH2), 1256 (C–O–C), 1070 (C–O), 830, 732 & 691 (aromatic C–H def); 1HNMR (CDCl3): δ (ppm) 8.32-7.08 (m, 13H, ArH), 7.02 (s, 1H, =CH–), 4.33-4.31 (t, 2H, HO–CH2–CH2–O–Ar), 3.58-3.56 (t, 2H, HO–CH2–CH2–O–Ar), 3.65 (s, 1H, O–H); MS, m/z (%): 402 [M+H]+ (100%). Anal.: Calcd. for C23H19N3O4: C, 68.82; H, 4.77; N, 10.47. Found: C, 68.89; H, 4.70; N, 10.41.
Antibacterial Activity
All the title compounds were screened for their in vitro antibacterial activity against two Gram positive strains, that is, Bacillus subtilis (MTCC 121) and Staphylococcus aureus (MTCC 96) and two Gram negative strains, that is, Escherichia coli (MTCC 40) and Pseudomonas aeruginosa (MTCC 2453), respectively. Ciprofloxacin was used as the standard drug for the present study. The serial two-fold dilution technique19 with slight modification20 was used for the study of antibacterial activity. A stock solution (10 µg/mL) of all the title compounds and standard drug was prepared in dimethyl sulfoxide. Sterilized double strength nutrient broth (DSNB) was used as a growth media. The stock solution was serially diluted by DSNB aseptically to give concentrations of 5.0–0.01 µg/mL into a series of sterilized culture tubes. All the tubes were inoculated by bacterial strain. The inoculum’s size was approximately 106colony forming units (CFU/mL). The inoculated tubes were incubated for 24 h at 37(±1)oC. After 24 h, the inoculated culture tubes were macroscopically examined for turbidity. The culture tube showing turbidity (lower concentration) and the culture tube showing no turbidity (higher concentration) gave the minimum inhibitory concentration (MIC) for the compound. The MIC for the title compounds and the standard drug, that is, ciprofloxacin are presented in Table 1.
Table 1: In vitro antibacterial activity of 1-phenyl-3-aryl-5-(4-(2-ethanoloxy) phenyl)-1H-pyrazoles (3a-g)
| Compound | Minimum Inhibitory Concentration (µg/mL) | |||
| B. subtilis | S. aureus | E. coli | P. aeruginosa | |
| (MTCC 121) | (MTCC 96) | (MTCC 40) | (MTCC 2453) | |
| 3a |
0.55 |
0.55 |
0.45 |
0.5 |
| 3b |
0.55 |
0.55 |
0.45 |
0.5 |
| 3c |
0.6 |
0.6 |
0.5 |
0.55 |
| 3d |
0.5 |
0.5 |
0.35 |
0.45 |
| 3e |
0.5 |
0.5 |
0.35 |
0.45 |
| 3f |
0.5 |
0.5 |
0.35 |
0.45 |
| 3g |
0.45 |
0.5 |
0.35 |
0.4 |
| Ciprofloxacin |
0.12 |
0.15 |
0.01 |
0.25 |
| (standard drug) | ||||
Results and Discussion
Chemistry
The synthesis of 1-phenyl-3-aryl-5-(4-(2-ethanoloxy) phenyl)-1H-pyrazoles were carried out according to the steps outlined in the scheme 1. Chalcones i.e., 1-aryl-3-(4-hydroxyphenyl) prop-2-en-1-ones, 1 were prepared by the reaction of 4-hydroxy benzaldehyde with substituted acetophenones following the Claisen-Schmidt reaction. The chalcones 1 then on refluxing with phenyl hydrazine in the presence of acetic acid and few drops of hydrochloric acid furnished 1-phenyl-3-aryl-5-(4-hydroxyphenyl)-1H-pyrazoles 2. Reaction of 2-chloroalkanols with 2 in the presence of triethyl amine gave the title compounds 3. All the compounds were obtained in good yield. These compounds were characterized on the basis of elemental and spectral analyses. IR spectra of each compound showed a band for O–H stretching vibrations for intermolecular hydrogen bonding near 3340 cm−1, while the C–O stretching vibrations for primary alcohols were observed in the range of 1070–1065 cm−1. The C–O–C stretching vibrations for aryl alkyl ether appeared at 1254 cm−1. The C–H stretching vibrations for methylene groups appeared in the range of 2916–2919 cm−1, whereas bending vibrations for methylene scissoring were observed constantly at 1465 cm−1. Aromatic C–H stretching vibrations were observed in the range of 3100–3050 cm−1, whereas aromatic C–H bending vibrations appeared below 900 cm−1. In case of 1H NMR, the chemical shift value for the O–H group was observed at about 3.65 δ (ppm) and appeared as singlet (s). Aromatic protons appeared as multiplet (m) in the range of 8.32–7.07 δ (ppm). The methine proton of the pyrazole nucleus absorbed at 7.02–7.01 δ (ppm) and appeared as singlet (s). The methylene protons adjacent to the O–H group [HO–CH2–CH2–O–Ar] appeared as triplet (t) in the range of 3.59–3.54 δ (ppm), whereas the methylene protons adjacent to the O–Ar group [HO–CH2–CH2–O–Ar] observed at 4.33–4.30 δ (ppm) and also appeared as triplet (t). Aromatic methyl and methoxy protons were observed at 2.34 δ (ppm) and 3.83 δ (ppm), respectively as singlet (s). All the title compounds showed [M+H]+of 100% intensity as the molecular ion peak. Compound containing chlorine showed isotopic peak at [M+2+H]+ of about 35% intensity to that of parent ion peak, whereas bromo derivative showed isotopic peak at [M+2+H]+ of about equal intensity. The results of elemental analyses were found in good agreement with the calculated values.
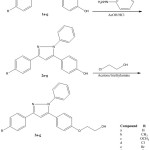 |
Scheme 1: Synthesis of 1-Phenyl-3-aryl-5-(4-(2-ethanoloxy)phenyl-1H-pyrazoles
|
Antibacterial Activity
All the synthesized title compounds were screened for their in vitro antibacterial activity against and two Gram positive bacterial strains, that is, Bacillus subtilis (MTCC 121) and Staphylococcus aureus (MTCC 96) and two Gram negative bacterial strains, that is, Escherichia coli (MTCC 40) and Pseudomonas aeruginosa (MTCC 2453), respectively, and their minimum inhibitory concentration (MIC) was determined. A perusal of the Table 1 shows that all the title compounds were found to be active against all the bacterial strains used in this study. However, they showed more activity against the Gram negative than the Gram positive bacterial strains. Out of the two Gram negative bacterial strains, E. coli (MTCC 40) was found to be more susceptible than P. aeruginosa (MTCC 2453) against all the title compounds. The minimum inhibitory concentration (MIC) of the title compounds 3a–g was found to be 0.55–0.45 µg/mL, 0.55–0.50 µg/mL, 0.45–0.35 µg/mL, and 0.50–0.40 µg/mL against B. subtilis (MTCC 121), S. aureus (MTCC 96), E. coli (MTCC 40) and P. aeruginosa (MTCC 2453) respectively. The MICs of the title compounds containing electron withdrawing groups like fluoro, chloro, bromo, or nitro were found somewhat less than the compounds containing electron releasing groups like methyl and methoxy. Compound 3g which contains nitro group was found to be most active amongst the title compounds. The reference standard ciprofloxacin inhibited Gram negative bacteria namley, E. coli and P. aeruginosa at a MIC of 0.01 µg/mL and 0.25 µg/mL, respectively, whereas against Gram positive bacteria namley, S. aureus and B. subtilis MIC was found to be 0.15 µg/mL and 0.12 µg/mL, respectively. The results of the MIC for the standard drug, ciprofloxacin, against the bacterial strains used were found to be within the range as reported in the literature21-23.
Conclusion
Present study describes the synthesis of a series of 1-phenyl-3-aryl-5-(4-(2-ethanoloxy) phenyl)-1H-pyrazoles starting from the chalcones. The compounds were characterized by modern analytical techniques such as CHN analyses, IR, Mass and proton NMR spectra. All the title compounds were screened for their in vitro antibacterial activity against Bacillus subtilis, Staphylococcus aureus (Gram positive) and Escherichia coli, Pseudomonas aeruginosa (Gram negative) and their minimum inhibitory concentration (MIC) were determined. The results of antibacterial activity showed that compounds containing electron withdrawing groups e.g., chloro, bromo, fluoro or nitro were found to be more active than the compounds containing electron releasing groups such as methyl and methoxy. These results suggest that some more compounds using different aromatic or heteroaromatic aldehydes, ketones, and haloalkanols should be synthesized and screened for their antibacterial activity to explore the possibility of 1-phenyl-3-aryl-5-(4-(alkanoloxy) phenyl) 1H-pyrazoles as a novel series of antibacterials.
Acknowledgments
The authors are thankful to the chairman of the Department of Pharmaceutical Sciences, Guru Jambheshwar University of Science and Technology, Hisar, India, for providing necessary facilities to carry out this work. Our sincere thanks are due to Department of SAIF, P.U. Chandigarh for elemental and spectral analysis. The director of IMTECH, Chandigarh, is also duly acknowledged for providing bacterial strains.
References
- Sharma R., Sharma C. L. and Kapoor B. Indian J. Med. Sci., 59 (3):120-129 (2005).
- Gomha S. M. and Hassaneen H. M. E., Molecules, 16 (8): 6549-6560 (2011).
- Abdel-Hafez E. M. N., Abuo-Rahma G. A. A., Abdel-Aziz M., Radwan, M. F. and Farag H. H., Bioorg. Med. Chem., 17 (11): 3829-3837 (2009).
- Ali T. E., Eur. J. Med. Chem., 44 (11), 4385-4546 (2009).
- Satheesha Rai N., Kalluraya B., Lingappa B., Shenoy S. and Puranic V. G., Eur. J. Med. Chem., 43 (8): 1715-1720 (2008).
- Witschel M., Bioorg. Med. Chem., 17 (12): 4221-4229 (2009).
- Lahm G. P., Stevenson T. M., Selby T. P., Freudenberger J. H., Cordova D., Flexner L., Bellin C. A., Dubas C. M., Smith B. K., Hughes K. A., Hollingshaus J. G., Clark C. E. and Benner E. A., Bioorg. Med. Chem. Lett., 17 (22): 6274-6279 (2007).
- Youssef A. M., White, M. S., Villanueva, E. B., El-Ashmawy I. M. and Klegeris A., Bioorg. Med. Chem., 18 (5): 2019-2028 (2010).
- Sauzem P. D., Machado P., Rubin, M. A., Sant’Anna G. S., Faber H. B., De Souza A. H., Mello, C. F., Beck P., Burrow R. A., Bonacorso H. G., Zanatta N. and Martins M. A. P., Eur. J. Med. Chem., 43 (6): 1237-1247 (2008).
- Abdel-Aziz M., Abuo-Rahma G. E. A., and Hassan A. A., Eur. J. Med. Chem., 44 (9), 3480-3487 (2009).
- Rostom S. A. F., Bioorg. Med. Chem., 18 (7): 2767-2776 (2010).
- Musad E. A., Mohamed R., Saeed B. A., Vishwanath B. S. and Rai K., Bioorg. Med. Chem. Lett., 21 (12): 3536-3540 (2011).
- Lowe I. and Southern J., Letters in Applied Microbiology, 18 (2): 115-116 (1994).
- Goyal A. and Jain S. Journal of Chemistry., Article ID 950491, http://dx.doi.org/10.1155/2013/950491 (2013)
- Goyal A. and Jain S., Der Chemica Sinica, 3 (1): 249-254 (2012).
- Goyal A. and Jain S., Der Pharma Chemica, 4 (1): 234-241 (2012).
- Abdel-Rahman A. A. H., Abdel-Megied A. E. S., Hawata, M. A. M., Kasem E. R. and Shabaan M. T., Monatsh Chem, 138 (9): 889-897 (2007).
- Voskiene A., Mickevicius V. and Mikulskiene G., Arkivoc, 15: 303-314 (2007).
- Cappucino J.G. and Sherman, N. Microbiology: A Laboratory Manual. Addison Wesley, San-Francisco, CA, 1999, pp 263-265.
- Jain S. Kumar A. Kumar M. and Jain N. Arab. J. Chem., published online: DOI: 10.1016/j.arabjc.2011.04.009 (2011).
- Bauernfeind A., J. Antimicrob. Chemother., 40 (5): 639-651 (1997).
- Hoogkamp-Korstanje J. A. A., J. Antimicrob. Chemother., 40 (3): 427-431 (1997).
- Weber D. J., Saviteer S. M., Rutala W. A. and Thomann C. A., Antimicrob. Agents Chemother., 32 (5): 642-645 (1988).

This work is licensed under a Creative Commons Attribution 4.0 International License.









