Synthesis and Structural Elucidation of Complexes of Some 3d-Series Divalent Transition Metals with 2-hydroxy-4-nitro acetophenone hydrazone, Schiff Base Ligand
Prem Mohan Mishra
Professor in Chemistry, M.L.S.M.College, Darbhanga, Bihar - 846 004, India.
DOI : http://dx.doi.org/10.13005/ojc/290241
The ligand 2 – hydroxy – 4 - nitro acetophenone hydrazone was synthesized by the condensation reaction.Complexes of Co(II), Ni (II) and Cu(II) was prepared by refluxing metal salts with the ligands.Properties of complexes have been studied by elemental analysis, study of solubility, decomposition temperature, magnetic susceptibility, electrical conductivity, electronic and IR spectra of ligands and complexes.On the basis of the experimental results probable structures of all the metal complexes have been proposed.
KEYWORDS:Synthesis; structure; complex compound 2 – Hydroxy – 4 – nitroacetophenone hydrazone; Co (II); Ni(II); Cu(II); Schiff’s base; Elemental analysis; Electronic spectra; IR spectra
Download this article as:| Copy the following to cite this article: Mishra P. M. Synthesis and Structural Elucidation of Complexes of Some 3d-Series Divalent Transition Metals with 2-hydroxy-4-nitro acetophenone hydrazone, Schiff Base Ligand. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Mishra P. M. Synthesis and Structural Elucidation of Complexes of Some 3d-Series Divalent Transition Metals with 2-hydroxy-4-nitro acetophenone hydrazone, Schiff Base Ligand. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22209 |
Introduction
Schiff’s bases are organic compounds having an azomethine group ( >C=N).Various studies1-2 have shown that >C = N group has considerable biological importance3-5. A broad spectrum of biological potential is reported to be associated with a number of Schiff’s bases6-8. Many evidences show that biological activity of a Schiff’s base enhances many fold on its co-ordination with suitable metal ions9-17. Due to this interesting structural features and important applications we have undertaken the study of synthesis and characterization of complexes of Co(II), Ni(II) and Cu(II) with Schiff’s base.
Experimental
Preparation of ligand
3 gms of hydrazine hydrochloride was dissolved in dil. HCl and 6 gms of 2 – Hydroxy – 4 – nitroacetophenone was dissolved in methanol. Both the solution were mixed together and refluxed for an hour. Yellowish coloured precipitate appeared on cooling the solution. The crude product was recrystallised from alcohol and melting point was recorded (M.P = 480 K). The pure dried compound was chemically analysed (Table – 1). The compound is soluble in alcohol but insoluble in acetone and water.
Preparation of complexes
Aqueous solution of chlorides of divalent metals (cobalt, nickel and copper) was prepared. Ligand was dissolved in methanol. Aqueous solution of metal chloride was mixed with methonolic solution of ligand and the mixture was refluxed on water bath for about three hours. The cold mixture was again refluxed with 2N ammonium hydroxide for an hour.On cooling the mixture metal complex is precipitated. The precipitate was washed dried and analysed chemically (Table – 1).
Table 1: Elemental Analysis Data of Ligand and Complexes
| Name of compound | SYMBOL OF ELEMENTS | ||||
| C | H | N | O | M | |
| Ligand (HNAPH) | 49.32(49.23) | 4.48(4.61) | 21.46(21.53) | (24.74(24.61) | — |
| [Co(HNAPH)3] | 44.48(44.51) | 3.65(3.71) | 20.42(20.40) | 22.25(22.26) | 9.20(9.12) |
| [Ni(HNAPH)3] | 44.83(44.92) | 3.65(3.74) | 19.38(19.65) | 22.94(22.46) | 9.20(9.23) |
| [Cu(HNAPH)2] | 42.45(42.52) | 3.50(3.54) | 18.42(18.60) | 21.53(21.25) | 14.10(14.06) |
Results
Solubility
All the complexes of cobalt, nickel and copper are soluble in DMF but insoluble in water. Cu(II) complex dissolves slightly in ethanol whereas complexes of cobalt and nickel are insoluble in methanol, ethanol and acetone.
Theoretical value is given in parenthesis
Table 2: Decomposition temperature, magnetic moment and electrical conductivity
| Complex | Decomposition temperature | Magnetic moment in (BM) | Molar electrical conductivity (Ohm-1) |
| [Co(HNAPH)3] | 573 K | 4.9 | 17.4 |
| [Ni(HNAPH)3] | 523 K | 3.01 | 18.9 |
| [Cu(HNAPH)2] | 513 K | 1.82 | 13.8 |
Table 3: Electronic spectral data of complexes
| Complex | Electronic spectra, d-d transition bands (in cm-1) | Assignment |
| [Co(HNAPH)3] | 9500 | 4T1g – 4T2g (F) |
| 19495 | 4T1g – 4A2g (F) | |
| 22315 | 4T1g – 4T1g (P) | |
| [Ni(HNAPH)3] | 11240 | 3A2g (F) – 3T2g (F) |
| 15995 | 3A2g (F) – 3T1g (F) | |
| 24685 | 3A2g (F) – 3T1g (P) | |
| 10750 | Spin forbidden transition | |
| [Cu(HNAPH)2] | 13845 – 14000 | 3Eg – 3T2g |
Table 4:
| Position of absorption bands (cm-1) | Assignment | |||
| Ligand(HNAPH) | [Co(HNAPH)3] | [Ni(HNAPH)3] | [Cu(HNAPH)2] | |
| 3420 | 3390 | 3400 | 3400 | NH2 stretching |
| 3195 | O – H (Hydrogen bonded) | |||
| 3140 | 3140 | 3140 | 3140 | C – H stretching |
| 3075 | 3080 | 3080 | 3080 | > N – H stretching |
| 2980 | 2980 | C – H (Aromatic) stretching | ||
| 2750 | C – H stretching | |||
| 2460 | 2460 | 2460 | 2460 | Skeletal vibrations |
| 2200 | 2200 | 2200 | 2200 | |
| 1910 | 1900 | 1900 | 1900 | |
| 16501620 | 16751630 | 16701625 | 16651635 | NH2, > N – H bending |
| 1590 | 1570 | 1565 | 1555 | C = N stretching |
| 1405 | 1395 | 1395 | 1395 | C – N stretching |
| 1260 | 1300 | 1290 | 1295 | C – O (Phenolic) |
| 920 | 920 | 920 | 920 | Characteristic vibrationsof 1,2,4 – tri – substitutedbenzene ring |
| 840 | 840 | 840 | 810 | |
| 780 | 780 | 780 | 785 | |
| — | 580 | 510 | 510 | M – N bond |
| 490 | 475 | 470 | M – O bond | |
Discussion
Structure of ligand
The interpretation of I. R. spectra is quite complicated due to the presence of various similar groups and hence many absorption bands. However, comparison of the spectral bands of the ligand (HNAPH), with those of its complexes (Table – 4), gives some important information regarding the nature of the ligand as well as the co-ordination sites through which metal ion co-ordinate with the ligand.
The broad band at 3195 cm-1 in the ligand assignable to phenolic O – H (hydrogen bonded) stretching frequency disappears in the Ni (II), Co(II) complexes showing deprotonation of phenolic protons through complexation18. The ligand also shows strong bands near 1260 cm-1 which may be attributed to the phenolic vibration. A shift of this band to higher frequency (1300,1290&1295 cm-1) in the complexes indicated chelation of the ligand to metal ion through phenolic oxygen.
The ligand exhibit a band near 3420 cm-1 and 3075 cm-1 which are assigned to NH2 and > N – H stretching19 respectively, does not change appreciably in the complexes indicating non – involvement of > N – H and NH2 nitrogen atom in chelate formation. Bands at 2460 cm-1, 2200 cm-1, 1900 cm-1, 920 cm-1, 840 cm-1 and 780 cm-1 are the characteristic bands of the compound which remain unaffected in complex formation.
In these cases of metal complexes, the most interesting feature noted is about the doublet band20 observed in the I. R. spectrum of the ligand near 1620 cm-1. The higher one goes upto 1650 cm-1 to 1675 cm-1 probably due to the bending mode of the NH2 group which remain practically unaffected and shows that the NH2 and > N – H mostly does not take part in the co-ordination. The other component which is due to C=NH group stretching frequency shifts to the lower frequency about (~1570 cm-1, 1565 & 1555 cm-1) in complexes, indicating the co-ordination through C = N (imine) group. In the case of Ni (II) and Cu(II) it has been observed that as the reaction is carried out at higher pH, co-ordination of Ni(II) and Cu(II) through C = N group, takes place by deprotonation. Martin and co-workers21 have also reported the same results.
A sharp band at 1590 cm-1 observable in the free ligand is assigned to C = N vibration of Schiff’s base residue. This band shifts to the lower frequencies in the complexes, which indicates that azomethine nitrogen of C = N takes part in co-ordination.
The due to nitro group, appearing in the region 1405 cm-1 in the free ligand, remains unaltered in all the complexes, suggesting non-participation of nitro group in co-ordination.
Thus 2 – Hydroxy – 4 – nitro acetophenone hydrazone (HNAPH) behaves as a bidentate ligand co-ordinating through phenolic oxygen ( C – O), nitrogen atom of C = N, of azomethine group and nitrogen atom of C=N, of imine group. It is further confirmed with experimental report.
It is noteworthy that the ligand behaves as monoprotonic for all the metal ions under investigation except for Ni (II) and Cu (II) when complexation is carried out at higher pH (pH = 9). In the later cases ligand behaves like biprotonic involving the deprotonation of C = NH (imine) proton. The structure of ligand may be represented as
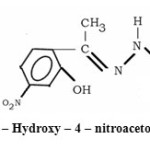 |
Figure 1 : 2 – Hydroxy – 4 – nitroacetophenone . Click here to View figure |
Structure of complexes
The diffuse reflectance spectra of cobalt complex gives three d-d transition bands which have been assigned to 4T1g (F) 4T2g (F), 4T1g (F) 4A2g (F) and 4T1g (F) 4T1g (P) and suggest an octahedral geometry22. Linkage of metal ions with the ligand through oxygen and nitrogen is further confirmed by the presence of I.R. bands. The room temperature magnetic moments of these Co(II) complexes around 4.9 BM suggest presence of three unpaired electrons and outer orbital octahedral structure for its complex. Molar conductance value suggest non-electrolytic nature of the complex. Taking all these facts into account an octahedral structure has been given to this complex.
Ni(II) complex show four bands in their reflectance spectra favouring an octahedral stereochemistry. Out of these four bands, three can be assigned to spin allowed d-d transition eg 3A2g (F) – 3T2g(F) ,3A2g (F) 3T1g (F) and 3A2g (F) – 3T1g (P).
The fourth band near 10750 cm-1 is probably spin forbidden and may be assigned to 2 B1g2 Eg transition.
The effective magnetic moment value (2.94 BM) are in good agreement with an octahedral geometry having two unpaired electrons.
Electrical conductivity data indicates non-electrolytic nature of nickel complex.
Copper (II) has configuration which makes Cu (II) subject to John – Teller distortion, if placed in an environment of cubic like regular octahedral or tetrahedral symmetry and this has a profound effect on all its stereochemistry23 -24.
The typical distortion is an elongation along one four fold axis, show that there is planar array of four short Cu-L bonds with two trans long ones. In the limit of course, the elongation leads to a situation indistinguishable from square co – ordination as found in many discrete complexes of Cu (II) . Thus the cases of tetragonally distorted “Octahedral” co – ordination and square co – ordination cannot be sharply differentiated.
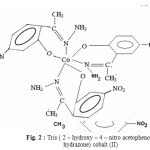 |
Figure 2 : Tris ( 2 – hydroxy – 4 – nitro acetophenone hydrazone) cobalt (II) |
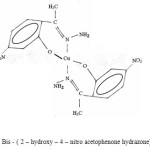 |
Figure 3 : Bis – ( 2 – hydroxy – 4 – nitro acetophenone hydrazone) Cu (II). |
Because of the relatively low symmetry of the environments in which the Cu2+ ion is characteristically found, detailed interpretation of spectra and magnetic properties are somewhat complicated even though one is dealing with the equivalent of a one – electron case25-28.
The complex [Cu (HNAPH)2], show a band at 2000 cm-1 in their electronic spectra, which suggest their square planar geometry, assigned to the overlap of 2B1g®2A1g, and 2B2g2Eg transitions29 .
The magnetic moment of these complexes correspond well with the presence of one unpaired electron and gives a specific information about their stereochemistries.
The molar conductance values suggest non – electrolytic nature of these complexes.Taking all these facts into consideration, along with their elemental analysis the most probable structures of these complexes are given below. These results are in conformity with our observations reported earlier.
 |
Graph 1Click here to View graph |
 |
Graph 2 : 2 – Hydroxy – 4 – nitroacetophenone. Click here to View table |
![Graph 3: [ Co(HNAPH)3], (b) : [Ni(HNAPH)3] , (c) : [Cu(HNAPH)2].](http://www.orientjchem.org/wp-content/uploads/2013/06/Vol_29-no2_Synt_Prem-grap3-150x150.jpg) |
Graph 3: [ Co(HNAPH)3], (b) : [Ni(HNAPH)3] , (c) : [Cu(HNAPH)2]. Click here to View garph |
References
- S. J. Gruber, C. M. Harris and E. Sinn : J Inorg Nucl. Chem, 30, 1805 (1968)
- M. Kato, K. Imai, Y. Muto, T. Tokki and H. B. Jonassen; J. Inorg. Nucl. Chem. 35, 109 (1973)
- M. Gulloli, L. Casello, A. Pasini and R. Ugo, J. Chem. Soc. Dalton Trans, 379 (1977)
- L. Muslim, W. Roth and H. H. Erlenmeyer: Acta Chem. Hev, 36,36 (1983)
- R. R. Vyas and R. N. Mehta: J. Ind. Chem. Soc. 68, 294 (1991).
- M. R. Manrao and V. K. Kaul, Indian J. Agric. Chem., 32(1990)33
- M. R. Manrao and Chander Kanta, V. K. Kaul, Indian J. Nematol, 27 (1997) 208
- R. H. Ububi and P. Purushottamachar, Indian J. Hetercyclic Chem., 9(2000) 189.
- K. D. Rainsford, M. W. Whitehouse, J. Pharm Pharmaco, 28 (1976) 83, Chem. Abstract, 85 (1976) 15392
- K. D. Domag, R. Buchnisch, F. Mietzsch and H. Schmidt, Naturewiss, Enschaften, 33 (1946) 315.
- F. P. Dwyer, F. Maythew and A. Shulman, Bril, J. Cancer, 19 (1965) 195.
- D. R. Williams, Chem, Rev. 72 (1972) 203.
- A. Furst and R. T. Haro, Progr. Exp. Tumor, Res., 12 (1969) 102.
- J. A. Gim and H. G. Patering, Cancer Res., 27 (1967) 1278.
- Z. Limermeister, B. Naturforsch, 5 (1950) 79.
- C. W. Johnson, J. W. Joyner and R. P. Perry, Antibiotics Chemotherpy, 2 (1952) 636.
- N. E. Morrison and F. M. Collins, Inst. J. Leprosy, 49 (1981)180.
- D. L. Leusing and B.L. Leach: J. Am. Chem. Soc. 60, 1098.
- R. H. Holm, G.W. Everett and A. Chakravorty : Prog. Inorg. Chem. 7, 161,(1966).
- M. S. Patil & J. R. Sah: J. Ind. Chem. Soc. 16, 944 (1981).
- R. B. Martin, M. Chamberlin and J. T. Chem. ; J. Am. Chem. Soc. 16, 944 (1981) Soc. 82, 495, (1960).
- M. S. Patil & J. R. Slah; J. Ind. Chem. Soc. 58, 944 (1981)
- W. E. Hatfield and R. Whymon : Transition metal chemistry 5, 47 (1969).
- B. J. Hathaway and D. E. Billing: Coordination Chem. Rev. 5, 143 (1970).
- F. A. Cotton and G. Willkinson; Advanced Inorganic Chemistry, Wiley Easterm Ltd. London Edn 7 (1984)
- J. R. Dyer: Applications of Absorption spectroscopy of organic compounds. Prentice Hall of India Pvt. Ltd. New Delhi (1974)
- R. S. Drago: Physical methods in Inorganic Chemistry. Affiliated East West Press Ltd. New Delhi, India (1978).
- K. Nakamoto, Infrared Spectra of Inorganic and Coordination compounds; John Willey International, New York (1970).
- I. M. Procter, B. J. Hathaway and P. A. Necholls: J. Chem. Soc. A, 1968 (1978).
- Shiv Shakti, P. M. Mishra, S. K. Mishra & A K Jha: Asian Journal of Chemistry. Vol. 22(7) 5013 – 5018.
- P. K. Jha, I. K. Jha and Prem Mohan Mishra: Asian Journal of Chemistry; Vol 17 (4) p – 2239-2242 (2005)
- B. K. Rai, Z. Hussain, W. P. Singh, S. N. Prasad, A. Prasad &P. M. Mishra: Journal of Ultra Chemistry; Vol 4(1) p – 53-60 (2008).
- B. K. Rai, S. Kumari, R. K. Singh, A. Prasad, M. P. Sinha &Prem Mohan Mishra: Journal of Ultra Chemistry: Vol. 5(1) p – 83 – 88 (2009).
- Shiv Shakti, Md. S. Q. Raze, D. K. Jha &P. M. Mishra; Journal of Ultra Chemistry Vol. 7(1) p- 113 – 122 (2011)
- Prem Mohan Mishra ; Journal of Ultra Chemistry Vol. 8(3) p. – 401 – 408 (2012).
- Prem Mohan Mishra; Journal of Ultra Chemisry Vol. 8(3) p.- 401 – 408 (2012)
- P. M. Mishra, D. K. Choudhary, Shiv Shakti, J. J. R. Chy. : Journal of Ultra Chemistry; Vol. 8(3) p – 415 – 420 (2012).

This work is licensed under a Creative Commons Attribution 4.0 International License.









