Synthesis and Characterization of Niobium (V) and Tantalum(V) Chelates with 2-aminothiophenol and 2-aminophenol
R. N. Pandey 1* and Pramila Sharma2
1P.G. Centre of Chemistry (M.U.), College of Commerce, Patna - 800 020, India.
2Department of Chemistry, Ganga Devi Mahila Mahavidyalaya, Patna - 800 020, India.
Corresponding Author E-mail: reachpramilaji@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/290240
Niobium(V) and Tantalum(V) Complexes [MLX4] (where M = Nb/Ta; X = Cl/NCS; L = 2-aminothiophenol or 2-aminophenol) have been synthesised and characterised on the basis of elemental analysis, conductivity measurements, Ir, UV-vis and ‘H NMR studies. The ligands behaves as mononegative bidentate donor. The value of magnetic moment of 0.35 – 0.43 BM is a consequence of combined effects of spin-orbit coupling and distortions of the ligand field from a full octahedral symmetry.
KEYWORDS:Nb(V); Ta(V); Six Coordinate; Iso-structural
Download this article as:| Copy the following to cite this article: Pandey R. N, Sharma P. Synthesis and Characterization of Niobium (V) and Tantalum(V) Chelates with 2-aminothiophenol and 2-aminophenol. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Pandey R. N, Sharma P. Synthesis and Characterization of Niobium (V) and Tantalum(V) Chelates with 2-aminothiophenol and 2-aminophenol. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22207 |
Introduction
Niobium (V) and Tantalum(V) are class-a metal but a NMR study of 93Nb has shown that the SCN ligand yields a series of N-bonded isothiocyanato and s-bonded thiocyanato complexes(1). These dº-complexes have no stereochemical preferences and exhibit a variety of coordination numbers(2-4) having very interesting insights into structure and bonding.(5-7) Some hexa-coordinate complexes with thioamide ligands are reported in our earlier paper.(8-10) The present study aims at synthesis and spectral characterization of some metal chelates with NS- and NO-donars 2- aminothiophenol and 2-aminophenol which are used as ligands by various workers.(11-13)
Experimental
Niobium and tantalum pentachlorides were obtained from Fluka and other chemicals 2-aminothiophenol (Schuchardt, Munchen),
2- aminophenol (Ward Blenkinsop, London) were CP grade. Solvents were dried and distilled before use. Niobium and tantalum were determined gravimetrically as Penta oxide and chloride as silver chloride. Nitrogen, Carbon and hydrogen were determined by microanalytical methods. The conductance of 10-3 M solutions of the complexes in DMF were measured using Wiss-Werkstatter Weithein obb type LBR conductivity meter. The IR spectra were recorded on a Perkin Elmer 577 spectrometer, electronic spectra on a Beckmann DV-6 spectrometer ‘H NMR (CDCl3) on a JE O2 JMS 60011 NMR spectrometer and magnetic moment was measured on a gouy balance using Hg[Co(SCN)4] as calibrant.
Synthesis of Nb(V) Complexes
Niobium (V) Chloride (0.01 mol) in chloroform (100 ml) was treated with a ligand (0.01 mol) in the same solvent. The reaction mixture was stirred on magnetic stirror for 1 hr and further refluxed for two hrs. on waterbath till evolution of HCl(g) ceases. Light yellow coloured solid were obtained on concentration. It was filtered under suction and washed with dry chloroform. The complexes were then dried and stored in vacuum over fused CaCl2(yield 78%).
Preparation of Isothiocyanato complexes of Niobium(V)
This complex was isolated with filterate of [Nb(S-C6H4-NH2)Cl4] following our previous method(10).
Preparation of Tantalum(V) complexes
All Tantalum(V) complexes were isolated similar to our previous method(10).
Analysis
Sl.No. 1 : For [Nb(S-C6H4NH2)Cl4]
Found(%): C, 20.20; H, 1.68; N, 3.52; Nb, 25.70, Calculated (%) : C, 20.11; H, 1.67; N, 3.91; Nb, 25.69;
Sl.No. 2 : For [Nb(O-C6H4NH2)Cl4]
Found(%) : C, 21.01; H, 1.75; N, 4.01; Nb, 26.80; Calculated (%):C, 20.99; H, 1.74; N, 4.08; Nb, 26.82;
Sl.No. 3 : For [Nb(S-C6H4NH2)(NCS)4]
Found(%) : C, 26.74; H, 1.35; N, 15.56; Nb, 20.69; Calculated (%):C, 26.72; H, 1.33; N, 15.59; Nb, 20.71;
Sl.No. 4 : For [Nb(O-C6H4NH2)(NCS)4]
Found(%) : C, 27.69; H, 1.35; N, 16.18; Nb, 21.42; Calculated (%):C, 27.71; H, 1.38; N, 16.16; Nb, 21.47;
Sl.No. 5 : For [Ta(S-C6H4NH2)Cl4]
Found(%) : C, 16.20; H, 1.35; N, 3.15; Ta, 40.50; Calculated (%):C, 16.10; H, 1.34; N, 3.13; Ta, 40.48;
Sl.No. 6 : For [Ta(O-C6H4NH2)Cl4]
Found(%) : C, 16.72; H, 1.90; N, 3.24; Ta, 41.95; Calculated (%):C, 16.70; H, 1.99; N, 3.24; Ta, 41.98;
Results and Discussion
The analytical data reveals a stoichiometry of 1:1, metal : ligand. The molar conductance value of 10-3 M solution in DMF were in the range of 16.60-30.90 ohm-1 cm-2 mol-1, indicating a non-electolytic behaviour of the complexes(14). The ligands behaves as mononegative bidentate and the formula of complexes may be proposed to be [ML X4] (M = Nb/Ta; LH = 2-aminothiophenol or 2-aminophenol; X = Cl/NCS). The value of paramagnetism between 0.35 BM- 0.50 BM is probably due to temperature independent second order Zeeman effect agreement with Fowles et al(15) and Nyholm and co-workers(16). The value of magnetic moment (0.35 BM- 0.43 BM) is assumed due to consequence of the combined effects of spin-orbit coupling and distortion of the ligand field from full octahedral symmetry.
Electronic and infrared Spectra
The electronic spectrum of 2-aminothiophenol exhibit two absorption maxima at 32260 cm-1 and 30485 cm-1, assignable to p®p* and n® p* transitions respectively. The strong absorption band at 27770 – 27700 cm-1 in the spectra of complexes is assigned to L®M charge transfer transition. No absorption bands are absorbed above 25000 cm-1 which indicates dº-configuration of complexes.
IR Spectra
A comparison of spectra of ligands and corresponding complexes indicate deprotonated ligands having Metal-S and Metal-N bonding with 2-aminothiophenol(ATP) and Metal-O and Metal-N bond with
2-aminophenol(AP). The uSH (2530cm-1) band of ATP and uOH (3480 cm-1) of AP were found to be absent from the spectra of complexes. The non-ligand bands at 315-320 cm-1 (ATP) and 680-685 cm-1 (AP) in complexes assigned to Metal-S and Metal-O stretching modes. The uasym(NH2)(3460cm-1) & usym(NH2)(3340cm-1) of free 2-aminothio-phenol (ATP) red shifts to lower frequency and observed at 3400-3360 cm-1 and 3300-3280 cm-1 respectively on complexation indicating coordination through amino nitrogen of ligand. This observation is further supported by non-ligand bands at 490-500 cm-1 of medium intensity and assigned to metal-N stretching modes(17). The new bands in the spectra of the complexes in the region 330-355 cm-1 (Nb) 446- 460 cm-1 (Ta) are assigned to uM-Cl modes(18-19).
N-bonded isothiocyanato group in complexes (Sl. No. 3 & 4) was confirmed by the new bands at 2040 cm-1(uCN), 785 cm-1 (uCS) and 490 cm-1 (d NCS) considering previous literature(20).
‘H NMR Spectra
The ‘H NMR spectra (CDCl3) of ligands and complexes were recorded to substantiate further mode of bonding. The free ligands, ATP and AP exhibits signals at d8.92 PPM and d3.68 PPM respectively due to intramolecularly hydrogen bonded phenolic and thiol protons. These protons disappeared from the spectra of complexes indicating their replacement by metal ion during complexation(21). The aromatic protons signals are observed in the range of d6.42 – 6.68 PPM(AP) and d6.64 – 7.30 PPM(ATP) as complex multiplet are slightly low field shifted and the integrated intensities of these signals agree well with the formulation of the complexes. The amino protons of ligands observed at d4.40 PPM (AP) and d3.68 PPM(ATP) are low field shifted on complexation and the integrated intensities of the signals agree well with the proposed structures of complexes (Str.- I).
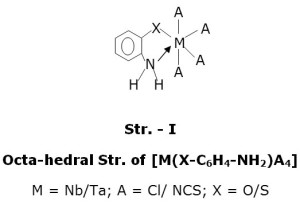
Table1: Major IR and ‘H NMR Spectral data of ligands and complexes.
|
Compds.
|
IR (cm-1) |
‘H NMR (dPPM) |
||||||
|
uasym NH2/ usym NH2) |
uM-N |
uM-O |
uM-S |
uM-Cl |
Amino Proton |
Phenyl Proton |
Thiol Proton |
|
| ATP (ligand) |
3460 m (3340 m) |
– |
– |
– |
355 m |
3.68 |
6.64-7.32 (multiplet) |
3.66 |
| (Sl. No. 1) |
3400 m (3300 m) |
490 m |
– |
320 w |
350 m |
3.54 |
6.62-7.10 (multiplet) |
– |
| (Sl. No. 3) |
3400 m (3310 m) |
495 m |
– |
310 w |
440 m |
3.55 |
6.42-6.68 (multiplet) |
– |
| (Sl. No. 5) |
3415 m (3310 m) |
500 m |
– |
315 w |
– |
3.58 |
6.40- 6.92 (multiplet) |
– |
| AP (ligand) |
3380 m (3300 m) |
– |
– |
– |
– |
4.40 |
6.42-6.68 (multiplet) |
– |
| (Sl. No. 2) |
3365 m (3285 m) |
480 m |
580 m |
– |
345 m |
4.11- 4.41 (splat) |
6.32 – 6.88 (multiplet) |
– |
| (Sl. No. 4) |
3360 m (3280 m) |
490 m |
590 m |
– |
335 m |
4.20 – 4.40 (splet) |
6.31 – 6.92 (multiplet) |
– |
| (Sl. No. 6) |
3370 m (3280 m) |
485 m |
685 m |
– |
460 m |
4.20 – 4.42 (splet) |
6.32 – 6.98 (multiplet) |
– |
References
- Kidel R.G. and Spinney H.G., J. Am. Chem. Soc. 103, 4759 (1981).
- Gilletti P.F., Femec D.A., Keen F.I. and Brown T.M., Inorg. Chem. 31, 4008 (1992).
- Barbara A.P., Fausto C. Ulli E., Caecilia M.M., Guido P. and Soachim S., J Chem. Soc. Dalton trans. 311(1996).
- Chabanur H.S., Revankar V.K. and Mahale V.B., Synth. React. Inorg. Met- Org. Chem. 31(2), 339 (2001).
- Rice D.A., Coord Chem. Rev. 37, 77(1981); 45, 87(1982).
- Holloway C.E. and Melnik M., Rev. Inorg. Chem. 7, 161 (1985).
- Holloway C.E. and Melnik M., J. Organomet. Chem. 303, 39(1986).
- Pandey R.N., Sharma R.N., Choudhary L.M.R. and Sharma (Mrs.) Pramila, J. Indian Chem. Soc. 69, 719(1992).
- Pandey R.N., Sharma R.N., Choudhary L.M.R. and Sharma (Mrs.) Pramila, Sahay A.N. and Sharma R.N. Indian J. Chem. 32A, 450 (1993).
- Pandey R.N., Sahay A.N., Sharma (Mrs.) Pramila, Kumar Sanjay and Pathak A.K., Asian J. Chem. Vol. 6(4), 747(1994).
- Singh B. and Choudhary R.V., Indian J. Chem. Vol. 13, 926 (1975).
- Larkworthy L.F., Murphy J.M. and Phillips D.J., Inorg. Chem. 7, 1436(1968).
- Wasson R. John, Wasson J. Shirley and Watermann M.G., Inorg. Chem. 9, 1576 (1970).
- Geary W.J., Coord. Chem Rev. 7, 81(1971).
- Fowles G.W.A., Tidmarst D.J. and Walton R.A., J. Chem. Soc. 1546(1969).
- Kepert D.L. and Nyholm R.S., J. Chem. Soc. 2871(1965).
- Gudasi Kala Gouda B., Maravalli P.B. and Goudar Rimmanagouda R., J. Serb. Chem. Soc. 70(4), 643(2005).
- Chabanur H.S., Revankar V.K. and Mahale V.B., SYNTH. REACT. INORG. MET. ORG. CHEM., 31(2), 339(2001).
- Puri D.M. and Singh S., J. Indian Chem. Soc. 58, 327(1981).
- Burmrister J.L., Coord. Chem. Rev. 1, 205 (1966); 3, 225(1968).
- Kutty K.Krishnan, Ummathur M.B. and Devi P. Sayu, J. Argent Chem. Soc. 96, 13(2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









