Synthesis and Antimicrobial activity of Some 1, 3, 4- Oxadiazoles-2-thione and 1, 2, 4-Triazoles Derivatives from Tert-Butyl Carbazate
Amira A. Ghoneim1 and Sahar A. Mohamed2
1Department of Chemistry, Faculty of Science, Zagazig University, Zagazig, Egypt.
2Biologie Technische Universität Braunschweig Mendelssohnstrasse 1D-38106 Braunschweig, Germany.
Corresponding author E-mail: aaghonium@zu.edu.eg
DOI : http://dx.doi.org/10.13005/ojc/290219
Tert-butyl carbazate (1) reacted with ammonium thiocyanate and phenyl isothiocyanate to afford the corresponding 5-tert-butoxy-4H-1, 2, 4-triazole-3-thiol (3) 5-tert-butoxy-4-phenyl-2H-1, 2, 4-triazole-3(4H)-thione 5 respectively. Compound (1) also reacted with carbon disulfide and potassium hydroxide to afford 5-tert-butoxy-1, 3, 4-oxadiazole-2(3H)-thione 6. Treatment of 6 with methyl iodide, 2, 3, 4, 6-tetra-O-acetyl-â-D-glucopyranosyl bromide and hydrazine hydrate to give the corresponding compounds (7, 8, 10) respectively. Reaction of 8 with ammonia gave the corresponding 9. Treatment compound 10 with 2-bromo-1-phenylethanone to give the corresponding 11. All new compounds were characterized by 1HNMR, IR Spectroscopy and elemental analysis. The biological activity of the prepared compounds was also described.
KEYWORDS:carbazate; phenylethanone; oxadiazole; 2-bromo-1-phenylethanone
Download this article as:| Copy the following to cite this article: Ghoneim A. A, Mohamed S. A, . Synthesis and Antimicrobial activity of Some 1, 3, 4- Oxadiazoles-2-thione and 1, 2, 4-Triazoles Derivatives from Tert-Butyl Carbazate. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Ghoneim A. A, Mohamed S. A, . Synthesis and Antimicrobial activity of Some 1, 3, 4- Oxadiazoles-2-thione and 1, 2, 4-Triazoles Derivatives from Tert-Butyl Carbazate. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22148 |
Introduction
The utility of hydrazides as key intermediates for the synthesis of several series of heterocyclic compounds and the broad spectrum of biological activities of their cyclized products have been reported1-4 and aroused our interest in exploring the utility of hydrazides as versatile precursors for the synthesis of a variety of substituted heterocycles,5-9 in addition to the interesting biological activities of thiazolo[ 3,2-a]benzimidazole derivatives such as antibacterial, 10,11 anti-inflammatory,12 antiulcer,13,14 antiviral15,16 and immunomodulatory17,18 activities.
Results and Discussion
In consideration of diverse biological properties of this type of compounds and in continuation of our interest in the synthesis of biologically active heterocyclic, the aim of the present work was to develop simple and efficient procedures for the preparation of new 1,2,4-triazole, 1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives. The synthesis of the 5-tert-butoxy-4H-1, 2, 4-triazole-3-thiol 3 was achieved in two steps from tert-butyl carbazate 1. The first step was the preparation of 4-carboxylic-acid-hydrazide-thioformamido-semi-carbazide 2 by treating the carbazate 1 with ammonium thiocyanate in dry benzene under reflux for 4 hrs. The resulting product after isolation showed an IR spectrum revealed absorption bands at 3100 cm-1 region for free and bonded NH another band at 1665 cm-1 (C=O) and at 1280 cm-1 (C=S). Mass spectrum showed a fragment [M+H] =191.25. The second step involved the treatment of 2 with sodium hydroxide in absolute ethanol under reflux for 5 hrs to give 3. IR spectrum exhibited the following characteristic bands 3200 cm-1 (NH), 2860-2760 cm-1 (SH) and 1670 cm-1 (C=N). 1H-NMR showed a singlet at 3.01 ppm for S-H. Mass spectrum exhibited a fragment [M+H] =173.24 attributed for C6H11N3OS. This information suggested that the triazole 3 found mostly in the thiol form.
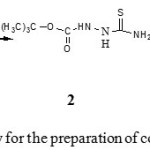 |
Scheme 1: Synthetic pathway for the preparation of compounds 2-3. Click here to View scheme |
Reaction of hydrazide 1 with phenyl isothiocyanate in absolute ethanol at reflux produced the corresponding hydrazinecarbothioamides 4 with good yields (Scheme 2).
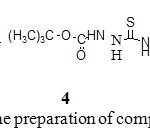 |
Scheme 2: Synthetic pathway for the preparation of compounds 4-5. Click here to View scheme |
The structure of hydrazinecarbothioamides 4 was confirmed by the spectral data. The IR spectrum of compound 4 displayed characteristic absorption bands in a region of 3235-3127 cm-1 for NH, at 1700-1692 cm-1 for C=O and in the region of 1348-1335 cm-1 corresponding to C=S vibrations. In the 1H NMR spectra three sets of NH group proton singlet as well as signals of phenyl group protons were observed confirming 4 formations. The cyclization of hydrazinecarbothioamides is an excellent for the synthesis of different heterocycles.19
The starting hydrazinecarbothioamides 4 on treatment with 10% sodium hydroxide solution at reflux undergo cyclodehydration to give 5-tert-butoxy-1,2,4-triazole-3-thiones 5. The structure was evident from spectral data. The IR spectrum displayed absorption bands at 3235-3127 cm-1 for NH, 1605-1595 cm-1 due to C=N group and at 1332-1330 cm-1corresponding to C=S stretch vibrations. The C=O group absorption was absent. The 1H-NMR spectra revealed far downfield signal of triazole NH proton at 12.89 ppm. The hydrazide 1 was refluxed with CS2 in absolute ethanol and KOH reveled into 5-tert-butoxy-1, 3, 4-oxadiazole-2(3H)-thione 6. The characteristic bands in IR of this compound showed at regions 1280 cm-1 (C=S) and 1675 cm-1 (C=N). The position of the C=N band suggested that the oxadiazole existed as the thione tautomer 6 rather than the ene-thiol form 6a which normally exhibited a band at a lower region (in about 1638 cm-1) due to maximum conjugation.20 Further support for the thione form came from 1H-NMR which exhibited only one proton as a singlet at lower field 7.01 ppm for N-H and no signal around 13 to 14 ppm where the S-H is normally shown.21 The product 6 also shown a fragment [M+H] = 203.04 in mass spectrometry. Sonication of compound 6 in the presence of methyl iodide in alkaline media afforded 2-tert-butoxy-5-(methylthio)-1, 3, 4-oxadiazole 7. Similarly, compound 6 reacted with tetra-O-acetyl-β-D-glucopyranosyl bromide in the presence of aqueous potassium hydroxide to give S-glycosylated through hydrogen bromide elimination and aromatization under the reaction conditions. The chemical formula and molecular structure of the formed glycosides 8 were identified using elemental analysis and spectra data. The 1H-NMR spectrums showed adoublet at 6.8 ppm assigned to the anomeric proton of the glucose moiety. The deacetylation of compound 8 was carried out in methanolic ammonia to get the corresponding deblocked nucleoside 2-(β-D-glucopyranosylthio)-5, 6, 7, 8-tetrahydro-4-(4-nitrophenyl) quinoazoline 9. The 4-amino-5- tert-butoxy-3H-1, 2, 4-triazole-3-thione 10 was synthesized by the reaction of 6 with hydrazine hydrate. Thus, treatment of compound 10 with 2-bromo-1-phenylethanone in refluxing ethanol yielded the 3-tert-butoxy-6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazine derivative 11 (Scheme 3). The latter products were established on the basis of their spectral data: For example, the lack of amino bands in their IR spectra and the appearance of the characteristic signals due to methylene protons of thiadiazine moiety in the region of 4.54-4.57 ppm.
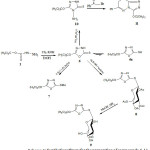 |
Scheme 3: Synthetic pathway for the preparation of compounds 6-11.
|
Antimicrobial Activity
The activity of the synthesized products was tested by the disk diffusion method. The cup-plate technique was used for the determination of these antimicrobial effects. Antibacterial and antifungal assays using Whatman No. 4 filter paper discs (0.5 cm diameter) were soaked in the tested sample. The samples were dissolved in DMSO (dimethyl sulfoxide). 0.24 μg of each sample was dissolved in 0.1 ml DMSO, then 0.1 ml of each sample was used with some gram positive bacteria such as (Sarcina lutea, Staphylococcus aureus and Bacillus subtilis) , gram negative bacteria such as (Pseudomonas aeuroginosa, Esherishia coli, Agrobacterium and Erwinia sp.) and fungi (Candida albicans, Aspergillus flavus) under aseptic conditions. The medium for cultivation of the test organisms was nutrient agar, and the Petri-dishes were incubated at 30 oC for 24 hrs. The results were obtained by measuring the inhibition zones (in mm) caused by the various compounds on the microorganisms. From the results, it is obvious that most of the tested compounds posses slight or no activity at all towards the tested microorganisms. However, some compounds showed considerable activity against the tested bacteria such as 3, 4, and 5. Others exhibit moderate or slight activity against the fungi such as 6, 7.
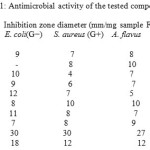 |
Table 1: Antimicrobial activity of the tested compounds |
* The concentration of the solution 20.0 mg/mL was tested; E. coli: Esherichia coli; G−: Gram negative bacteria; S. aureus: Staphylococcus aureus; G+: Gram positive bacteria; A. flavus: Aspergillus flavus; C. albicans: Candida albicans
Experimental
General Procedures
Melting points were determined with an Electro Thermal Mel-Temp II apparatus and are all uncorrected. IR spectra were obtained in the solid state as a potassium bromide disc using a Perkin-Elmer model 1430 Spectrometer. 1HNMR were recorded on aVarian/Gemini 200/MHZ spectrometer in DMSO-d6 as a solvent and TMS as an internal standard (chemical shift in δ, ppm). Mass spectra were measured on an instrument “VG-7035”. Spectra were recorded at 70 or 15 electron volt. Elemental analysis was performed at the Micro analytical centre, CairoUniversity, Giza, Egypt.
N-Tert-Butyl Formate- Thiocarbonohydrazide
The hydrazide 1 (160 mg, 0.01 mol) was added to ammonium thiocyanate (0.01 mol) in dry benzene (10 ml) and the mixture was heated under reflux for 6 h (TLC (CHCl3 / MeOH, 6:4), Rf: 0.60).The solid material obtained on cooling was filtered and recrystallized from methanol (0.58 g, 85 %), mp 145-147 °C. IR cm-1: 3100 (NH), 1665 (CON), 1280 (C=S).1H- NMR (250 MHz, DMSO-d6) δ (ppm):1.41 (s, 9H, 3CH3), 2.00 (s, 1H, NH), 2.04 (s, 2H, NH2), 8.01 (s, 1H, N-NH). Anal. Calcd for C7H13N3O3S: (191.25); C, 37.68; H, 6.85; N, 21.97; S, 16.77. Found: C, 37.68; H, 6.65; N, 21.72; S, 16.34.
5-Tert-Butoxy-4H-1, 2, 4-Triazole-3-Thiol
The compound 2 (220 mg, 10 mmol) in 2N-NaOH solution (40 mg NaOH, 10 mmol in 100 ml H2O) was heated under reflux for 5 h. (TLC (CHCl3/ MeOH, 6:4); Rf: 0.55). After cooling, the solution was made acidic with conc. HCl and the precipitate was then recrystallized from absolute ethanol to give crystalline fibers 11 (295 mg, 84 %). IR cm-1:3200 (NH), 2860-2760 (S-H), 1670 (C=N). 1H NMR (250 MHz, DMSO-d6) δ (ppm): 1.41 (s, 9H, 3CH3), 3.00 (s, 1H, C-SH), 3.63 (s, 1H, NH), Anal. Calcd for C6H11N3OS: (173.24); C, 41.60; H, 6.40; N, 24.26; S, 18.51 found: C, 41.66; H, 6.49; N, 24.46; S, 18.34
3.4. N-Tert-Butyl Formate-4-Phenylthiosemicarbazide
A mixture of hydrazide 1 (2.83 g, 10 mmol) and phenyl isothiocyanate (11 mmol) in abs. ethanol (50 ml) was refluxed for 3 h and then cooled to room temperature. The resultant white solid was collected by filtration, dried and crystallized from ethanol. Yield 84%, mp 191-193 °C; 1H NMR (DMSO-d6), δ, ppm: 1.62 (s, 9H, 3CH3), 7.18-7.30 (m, 5H, phenyl), 9.50 (s, 1H, NH-Ph), 9.75 (s, 1H, NHCS), 10.29 (s, 1H, CONH); Anal. Calcd: for C12H17N3O2S2 (267.35); C, 53.91; H, 6.41; N, 15.72; S, 11.99. Found: C, 53.96; H, 6.36; N, 15.69; S, 11.93.
5-Tert-Butoxy-4-Phenyl-2H-1, 2, 4-Triazole-3(4H)-Thione
A mixture of compound 4 (1 mmol) and 10% sodium hydroxide solution (20 ml) was refluxed for 3 h, cooled to room temperature, poured over crushed ice and acidified with conc. HCl to pH 5. The precipitate was collected by filtration, washed with water, dried and crystallized from ethanol. Yield 85%, mp 198-200°C; 1H NMR (DMSO-d6), δ, ppm: 2.18 (s, 9H, 3CH3), 7.41-7.44, 7.51- 7.53 (2m, 5H, phenyl), 12.89 (brs, 1H, NH); Anal. Calcd. For C12H15N3OS: (249.33); C, 57.81; H, 6.06; N, 16.85; S, 12.86. Found: C, 57.78; H, 6.26; N, 16.80; S, 12.81.
5 -Tert-Butoxy-1, 3, 4-Oxadiazole-2 (3H)-Thione
The hydrazide 7 (300 mg, 0.30 mol) was dissolved in absolute ethanol and CS2 (3 ml, 0.05 moles) and KOH (1.8 g, 0.3 moles in H2O, 20 ml) were added. The reaction mixture was heated under reflux with an aid of magnetic stirring for 3 h, during that time the color of the solution turned to yellow (H2S evolution took place during the reaction time. TLC (CHCl3 / MeOH, 6:4), Rf: 0.65). Ethanol was distilled off under reduced pressure and the residue was dissolved in H2O, acidified with HCl to pH=2 and extracted with EtOAc (3 x 20 ml). The combined extracts were evaporated to dryness under vacuum to give crystalline solid 8 (0. 275 g, 82 %), mp 165-167 °C. IR cm-1: 1675 (C=N), 1150 (C-O-C), 1280 (C=S). 1H -NMR (250 MHz, DMSO-d6) δ (ppm):1.42 (s, 9H, 3CH3), 7.01 (s, 1H, NH). Anal. Calcd. For C6H10N2O2S (174.22): C, 41.36; H, 5.79; N, 16.08; S, 18.40. Found: C, 41.40; H, 5.83; N, 16.28; S, 18.37.
2-Tert-Butoxy-5-(Methylthio)-1, 3, 4-Oxadiazole
Compound 6 (1.58 g, 6.1 mmol) was dissolved in mixture of ethanol (4 ml) and 10% aqueous sodium hydroxide solution (2.75 ml). Methyl iodide (0.44 ml, 7 mmol) was added and the solution was sonicated for 20 min. Water (20 ml) was added to the reaction mixture and extracted with ether. The organic layer was washed with water and dried over sodium sulfate, filtered, and concentrated in vacuo. The residue was purified by column chromatography eluting with petroleum ether-ethyl acetate (4:1) and then crystallized from methanol to give 1.41 g (85%) of 11a; mp 44–46 C. IR (KBr): ν 3073cm–1(aromatic).1H NMR (CDCl3): δ 2.77 (s, 9H, 3CH3). Anal. Calcd. C7H12N2O2S (188.25): C, 44.66; H, 6.43; N, 14.88; S, 17.0. Found: C, 44.59; H, 6.39; N, 14.78; S, 17.23.
2-Tert-Butoxy-5-(2`,3`,4`,6`-Tetra-O-Acetyl-Β-D-Glucopyranosylthio)-1,3,4-Oxadiazole
To a solution of 5-tert-butoxy-1, 3, 4-oxadiazole-2 (3 H)-thione 6 (0.23 g, 0.79 mmole) in aqueous potassium hydroxide (0.73 g in 10 ml of distilled water), was added a solution of 2, 3, 4, 6-tetra-O-acetyl-β-D-glucopyranosyl bromide (0.46 g, 2.0 mmole) in acetone (20 ml). The reaction mixture was stirred at room temperature until the reaction complete, then evaporated under reduced pressure and the residue was washed with distilled water to remove the formed potassium bromide. The product was dried and recrystallized from ethanol to afford pale yellow crystals, yield 63%, m.p. 206-211°C. IR (KBr): 1736 ,1604 , 1517 ,1325, 523 cm-1; 1H-NMR δ 7.61-8.19 (d,d , 4H, C6H4), 6.82 (d, 1H, H-1`), 5.12 (m, 2H, H-6`,6“), 4.32- 4.47 (m, 4H, H-2`,3`,4`,5`), 3.17 (t, 4H, 2CH2), 2.46 (m, 4H, 2CH2), 1.90-2.13 (4s, 12H, 4OAc) ppm; M.S: (M+ 617.17) which is corresponding to molecular formula C28H31N3O11S. Anal. Calcd for C20H28N2O11S (504.51): C, 47.61; H, 5.59; N, 5.55; S, 6.36. Found: C, 47.66; H, 5.62; N, 5.53; S, 6.42.
2-Tert-Butoxy-5-(Β-D-Glucopyranosylthio)-1, 3, 4-Oxadiazole
(2S, 3S, 4S, 5S)-2-(5-tert-butoxy-1, 3, 4-oxadiazol-2-ylthio)-tetrahydro-6-(hydroxymethyl)-2H-pyran-3, 4, 5-triol (9)
A solution of nucleoside 8 (1 mmole), in absolute methanol (10 ml) was added at 0oC to a saturated solution of anhydrous NH3 in absolute methanol (25 ml) and the mixture was stirred at 0oC for 4 hrs., and then at room temperature for an additional 12 hrs, purified on a silica gel column (MeOH: CHCl3, 1:9, v/v) and then crystallization from methanol gave compound 9 yield 65%, m.p. 175-181oC. IR (KBr): 3400-3521, 1621, 1525, 1352, 551cm-1; M.S: (M+, 449.13) which is corresponding to molecular formula C20H23N3O7S has fragmentation ions at m/e at (224, 100%) and (322, 7.0%) Anal. Calcd for C12H20N2O7S (336.36): C, 42.85; H, 5.99; N, 8.33; S, 9.53. Found: C, 42.91; H, 5.93; N, 8.38; S, 9.49.
4- Amino-5- Tert-Butoxy-3H-Triazole-3-Thione
To a suspension of 6 (0.4 g, 1.4 mmol) in ethanol (2 ml), hydrazine hydrate (0.14 ml, 2.8 mmol) was added. The reaction mixture was heated at reflux for 4 h, cooled and poured into crushed ice. The resulting solid was collected by filtration, washed with water and crystallized from ethanol to give 50 mg (11%) of 12, mp 138–140 C. IR (KBr): ν 3432, 3336 (NH2), 3250(NH), 1325 (C=S). 1H-NMR (CDCl 3): δ 13.40 (bs, 1H, NH), 7.46–7.403 NH), 4.90 (bs, 2H, NH2). Anal. Calcd. For C6H12N4OS (188.25); C, 38.28; H, 6.43; N, 29.76; S, 17.03
3-Tert-Butoxy-6-Phenyl-7H-[1, 2, 4]Triazolo[3,4-B][1,3,4]Thiadiazine
A mixture of compound 10 (0.60 g, 2 mmol) and the appropriate haloketones (2 mmol) in ethanol (30 ml) was refluxed for 8 h. The formed precipitate was filtered off, washed with ethanol and dried. Recrystallization from EtOH/DMF gave compounds 11. m.p 307–310 oC. IR (KBr): ν 1583 (C=N).1H-NMR (CDCl3): δ 2.23 (s, 9H, 3CH3), 4.34 (s, 2H, thiadiazine), 7.32-7.56 (m, 5H, phenyl). Anal. Calcd. For C14H16N4OS (288.37): C, 58.31; H, 5.59; N, 19.43; O, 5.55; S, 11.12. Found: C, 58.37; H, 5.62; N, 19.46; O, 5.50; S, 11.22.
Conclusions
We have established a new and efficient synthesis of a novel series of 1, 3, 4-oxadiazoles-2-thione and 1, 2, 4-triazoles derivatives from tert-butyl carbazate. We could also extend this technique to the synthesis of new 2-Tert-butoxy-5-(β-D-glucopyranosylthio)-1, 3, 4-oxadiazole containing sugar moiety. The antifungal and antibacterial activities of the newly synthesized compounds were evaluated.
Acknowledgements
The author is grateful to ZagazigUniversity and the Deanship of Scientific Research. He also offers his thanks to the Faculty of Science and Department of Chemistry for their support.
References
- Tozkoparan, B.; Gökhan, N.; Aktay, G.; Yesilada, E.; Ertan, M. 6-Benzylidenethiazolo[3,2-b]-1,2,4-triazole-5(6H)-ones substituted withibuprofen: synthesis, characterization and evaluation of anti-inflammatory activity Eur. J. Med. Chem. 2000, 35, 743.
- Demirbas, N.; Ugurluoglu, R.; Demirbas, Synthesis and Antimicrobial Activities of Some New 1,2,4-Triazole Derivatives A. Bioorg. Med. Chem. 2002, 10, 3717.
- Holla, B. S.; Akberali, P. M.; Shivananda, M. K. Studies on nitrophenylfuran derivatives part Xii. synthesis, characterization, antibacterial and antiviral activities of some nitrophenylfurfurylidene-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines. Farmaco 2001, 56, 919-927.
- Holla, B. S.; Kalluraya, B.; Sridhar, K. R.; Drake, E.; Thomas, L. M.; Bhandary, K. K.; Levine, M. J. Ethyl 5-[6-(furan-2-yl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazol-3-yl]-2,6-dimethylnicotinate. Eur. J. Med.Chem. 1994, 29, 301-308.
- Dawood, K. M.; Farag, A. M.; Abdel-Aziz, H. A. A Convenient Access to Functionalized Pyrazole, Pyrazolyl-azole, and Pyrazolo [3, 4-d]pyridazine Derivatives J. Chin. Chem. Soc. 2006, 53, 873-880.
- Dawood, K. M.; Farag, A. M.; Abdel-Aziz, H. A. N-[Phenyl (piperidin-1-yl) methyl]-1, 3, 4-thiadiazol-2-amine. Heteroatom Chem. 2005, 16, 621.
- Dawood, K. M.; Farag, A. M.; Abdel-Aziz, H. A. Facile route to novel 2-pyridone, pyrazolo[3,4-d]-1,2,3-triazine, and pyrazolo[3,4-d]- and [1,5-a]-pyrimidine derivatives Heteroatom Chem. 2007, 18, 294.
- Farag, A. M.; Dawood, K. M.; Abdel-Aziz, H. A. (Z)-Ethyl 2-cyano-2-{2-[5,6-dimethyl-4-(thiophen-2-yl)-1H-pyrazolo[3,4-b]pyridin-3- yl]hydrazinylidene}acetate J. Chem.Res. 2004, 808-810.
- Dawood, K. M.; Farag, A. M.; Abdel-Aziz, H. A. J. Chem. Res. 2005, 278.
- Allanson, N. M.; Leslie, B. W.; Thomson, S. Brit. UK Pat Appl GB 2,376,944 2001; [Chem. Abstr., 138, 55966t (2003)].
- Oh, C.; Ham, Y.; Hong, S.; Cho, Synthesis and Antibacterial Activity of New 1β-Methyl carbapenem Having a Thiazolo[3,2-a]benzimidazole Moiety. J. Arch. Pharm. 1995, 328, 289- 291.
- Bender, P. E.; Hill, D.; Offen, P. H.; Razgaitis, K.; Lavanchy, P.; Stringer, O. D.; Sutton, B. M.; Giswold, D. E.; Dimertino, M. 5,6-Diaryl-2,3-dihydroimidazo[2,1-b]thiazoles: a new class of immunoregulatory antiinflammatory agents. J. Med. Chem. 1985, 28, 1169-1177.
- Yoshida, A.; Oda, K.; Tabata, K. Jpn. Kokai Tokkyo Koho JP 03 14,566 1991; [Chem. Abstr., 115, 71660z (1991)].
- Crossley, R. U.S. US 4,873,237 1989; [Chem. Abstr., 112, 198401d (1990)].
- Moormann, A. E.; Becker, D. P.; Flynn, D. L.; Li, H.; Villamil, C. I. Synthesis and Reactions of 3-Methylthiazolo[3,2-a]benzimidazole- U.S. US 5,945,425 1999; [Chem. Abstr., 131, 184948t (1999)].
- Moormann, A. E.; Becker, D. P.; Flynn, D. L.; Li, H.; Vilamil, C. I. Synthesis of Some 1,3-Thiazole, 1,3,4-Thiadiazole, Pyrazolo[5,1-c]-1,2,4-triazine, and 1,2,4-Triazolo[5,1-c]-1,2,4-triazine Derivatives Based on the Thiazolo[3,2-a]benzimidazole Moiety PCT Int. Appl. WO 95 29,897 1995; [Chem. Abstr., 124, 202255b (1996)].
- Gilman, S. C.; Carlson, R. P.; Daniels, J. F.; Datko, L.; Berner, P. R.; Chang J.; Lewis, A. Immunological abnormalities in rats with adjuvant-induced arthritis–II. Effect of antiarthritic therapy on immune function in relation to disease development. J. Int. J. Immunopharmacol 1987, 9, 9-16.
- Tagliabue, A.; Allesandri, G.; Polentarutti, N.; Mantovani, A.; Falantano, E.; Veccchi, A.; Garattini, S.; Spreafico, F. Synthesis and Reactions of 3-Methylthiazolo[3,2-a]benzimidazole-2-carboxylic Acid Hydrazide: Synthesis of Some New Pyrazole, 1,3-Thiazoline, 1,2,4-Triazole and 1,2,4-Triazolo[3,4-b]-1,3,4-thiadiazine Derivatives Pendant to Thiazolo[3,2-a]benzimidazole Moiety Eur. J. Cancer. 1978, 14, 393
- (A) Verma, A.; Saraf, S. K. 4-thiazolidinone–a biologically active scaffold Eur. J. Med. Chem. 2008, 43, 897-905.
- Kucukguzel, G.; Kocatepe, A.; Clercq, E.; Sahin, F.; Gulluce, M. Synthesis and biological activity of 4-thiazolidinones, thiosemicarbazides derived from diflunisal hydrazide. Eur. J. Med. Chem. 2006, 41, 353-359.

This work is licensed under a Creative Commons Attribution 4.0 International License.









