Synthesis and Antimicrobial Activity of 2-(2- ketothiophen-yl)-3-(substituted aryl)- 5-{methylene-(4-haloaryl)}-1- thiazolidin-4-ones
Geeta Raghav, Sanidhya Upadhyay and R. K. Upadhyay*
1Department of Chemistry, N. R. E.C.College, Khurja, India.
2C. C. S. University Meerut, India.
Corresponding Author E-mail: rkupadhyay@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/290232
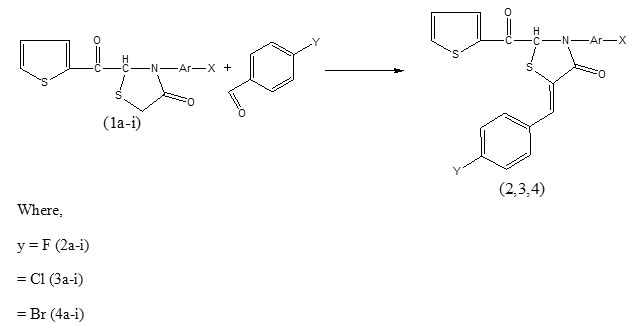
New 2-(2-ketothiophen-yl)-3-(substituted aryl)-5-{methylene-(4-haloaryl)}-1-thiazolidin-4-ones synthesized by condensation of 2-(2-ketothiophen-yl)-3-(substituted aryl) -1-thiazolidin-4ones with p-fluoro, p-chloro- and p-bromo- benzaldehydes were characterized by their elemental analyses, molecular weight determination and 1R and 1HNMR spectroscopy. Some of the synthesized compounds have been screened in vitro for their antimicrobial activity against B. subtilis, S. aureus, E. coli, and P. aeruginosa bacteria and C. albicans and A. niger fungi. Almost all the tested compounds displayed pronounced biological activities
KEYWORDS:Antimicrobial Activity; Bacteria; Fungi
Download this article as:| Copy the following to cite this article: Raghav G, Upadhyay S, Upadhyay R. K. Synthesis and Antimicrobial Activity of 2-(2- ketothiophen-yl)-3-(substituted aryl)- 5-{methylene-(4-haloaryl)}-1- thiazolidin-4-ones. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Raghav G, Upadhyay S, Upadhyay R. K. Synthesis and Antimicrobial Activity of 2-(2- ketothiophen-yl)-3-(substituted aryl)- 5-{methylene-(4-haloaryl)}-1- thiazolidin-4-ones. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22186 |
Introduction
Among the wide range of small ring heterocycles that containing nitrogen and sulphur have been under investigation for pretty long time because of their multifarious medicinal potential especially as antimicrobials. A survey of literature has revealed that 4-thiazolidinones possessing thiazole nucleus have been shown to have manifold biological activities such as antiviral, anti- HIV, anticancer, anticonvulsant, analgesic, anti-inflammatory, antihistaminic, antituberculostatic, antibacterial, antifungal etc, properties1-12. Although plenty of these versatile molecules with variety of substituted groups at position 2, 3and 5 of thiazolidinone nucleus have been synthesized and screened for diverse biological properties but majority of reports pertain to their antimicrobial evaluation in search of new potential antimicrobial agents with a zeal to address the long persisting problem of drug resistance of some bacteria and fungi. The high biological significance in general and antimicrobial potential of this class of compounds in particular impelled us to synthesize new thiazolidinone derivatives by condensation of 2-(2-ketothiophen-yl)-3-(substituted aryl) -1-thiazolidin-4ones with 4-fluoro-, 4-chloro- and 4-bromo- benzaldehydes in continuation to our previous work 13-15 and to screen some of them as typical examples in vitro for antimicrobial activities against some Gram-positive and Gram-negative bacteria and fungi.
Experimental
General Procedure for the Synthesis of Compounds
Preparation of 2-(2-ketothiophen-yl)-3-(substituted aryl) -1-thiazolidin-4-ones
All the title compounds were synthesized by cyclocondensation of ketoazomethiones, obtained by condensation of 2-thiophene glyoxal and primary amines, with thioglycolic acid as reported earlier16.
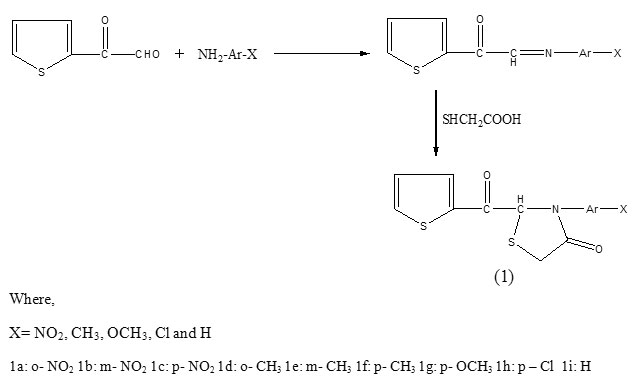
Physico-chemical and antimicrobial analyses
Elemental analyses for carbon, hydrogen, nitrogen and sulphur contents of the synthesized compounds were performed on a variro El-III elemental – R analyzer. Melting points determined in open glass capillaries were uncorrected. Molecular weights of the compounds were determined by Rast’s method using camphor as solvent. IR spectra were recorded in KBr medium in 4000-500 cm-1 range on Thermo Nicolet Nexus FT-IR spectrometer. 1H NMR spectra were recorded in dimethylsulphoxide medium on Bruker-400 Mhz spectrophotometer.
Antimicrobial activity against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa bacteria and Candida albicans and Aspergillus niger fungi was determined by the micro broth dilutions technique. Mueller-Hinton broth was used as the test medium. Serial two-fold dilutions from ranging from 400µg/ml to 25µg/ml in DMSO-water (1:4, v/v). Mueller-Hinton broth was used as nutrient medium to grow and dilute the drug suspension. DMSO was used as diluents. The inoculum was prepared using a 4-6 h broth culture for each bacteria and 24 h culture for fungi strains adjusted to a turbidity equivalent to a 0.5 to 0.6 optical density diluted to broth media to give a final concentration in Elissa plate. The plates were covered with sterilized aluminium foil to prevent evaporation and incubated at 35-370C for 24 h for bacteria and 48 h for fungi. The minimum inhibitory concentrations (MIC), defined as the lowest concentration of the compound giving complete inhibition of visible growth, were determined. Ampicillin(standard antibiotic) and griseofulvin ( standard antifungal drug) were as positive controls for antimicrobial and antifungal screening.
results and discussion
Analytical and physical data of the synthesized compounds are noted in table1. Theoretically proposed molecular formulae of the products are in fair agreement with their elemental analyses and molecular weight data.
The condensation of 1a-i with p-halogen substituted benzeldehydes involving methylene (CH2) group of thiazolidinone ring (1a-i) and aldehyde (CHO) group of benzaldehydes leads to the formation of a new >C = CH – C< functional moiety between thiazolidinone ring carbon and CH of aldehyde group of benzaldehydes with the release of a water molecules whereas other groups of the reactants remained intect. The infrared spectra of the synthesized compounds displayed all the frequencies of characteristic groups of 1a-i, and 2a-i, 3a-i and 4a-i, υC=O, υC-N, υC-S-C of pentacycle thiazolidinone ring and its adjacent C=O group υC=O (chain) and υC=C & υC-H of benzene ring in 1666-1769cm-1, 1298-1367cm-1, 663-736 cm-1, and 1605-1669 cm-1 and 1441-1603 cm-1 & 2995-3113cm-1 regions17 respectively except bands corresponding to CH2 of thiazolidinone ring (1a-i) and C=O of aldehydes (2a-i, 3a-i and 4a-i). The absence of these bands of reactants and appearance of two new bands in 1804-1949 cm-1 and 2893-2955 cm-1 regions attributed to υC=CH- and υC-H respectively clearly indicate condensation of methylene group of 1a-i with carbonyl group of aldehydes. Bands corresponding to ortho, meta and para disubstitution in benzene ring appeared in 733-768 cm-1, 745-761 cm-1 and 810-842 cm-1 regions respectively in the spectra of the products. In order to confirm infrared results regarding condensation of the mentioned reactants 1H NMR spectra of nine compounds (2c, 2f, 2g, 3c, 3f, 3g, 4c, 4f and 4g) as typical examples have been examined. The absence of chemical shift corresponding to methylene (CH2) group 2H of thiazolidinone ring and appearance of a new singlet peak in δ 1.53-2.50 ppm range attributed 18 to >C = CH – C< 1H strongly support IR inferences. Benzene and thiophene ring protons displayed their chemical shifts in δ 6.56-7.98 ppm region.
It is observed (Table2) that all the compounds irrespective to the nature of substituted groups in both benzene rings are better bactericides and fungicides than standard reference drugs in general except unsubstituted aniline product (3i). Compounds 2g, 3e and 4g with high antifungal activities could be assumed as selective drugs for both of the test fungi. Almost same antibacterial and antifungal activities of 2e and 3e containing fluoro and chloro substitutents with common m-OCH3 group, and 3a and 4a containing chloro and bromo substitutents with common o-NO2 group may be due to small differencein electronegativities of halogen substitutents in each pair of compounds. However the effect of electronegativity of halogen substitutents is more pronounce in exhibiting antibacterial activities as has been observed in 2g and 4g containing fluoro and bromo substitutents with common OCH3 group. Compound 2g having more electronegative halogen substitutent, fluoro, is better antibactericide than 4g of bromo substitutent. Among all the compounds however 2g is the best in exhibiting both, antibacterial and antifungal activities against all the microbes.
Table 1: Colour, melting point, yield, molecular weight, and analyses data of compounds.
|
Compound |
Colour |
Yield (%) |
M.P. (0C) |
M.W. Calcd. (Found) |
Analyses (%): Calcd.(Found) |
|||
|
C |
H |
N |
S |
|||||
| 2a | Lemon | 42 | 185 | ― | 57.27(57.05) | 2.95(2.93) | 6.36(5.90) | 14.54(14.10) |
| 2b | Yellow | 45 | 176 | 440(441.1) | 57.27(56.78) | 2.95(2.66) | 6.36(6.11) | 14.54(14.45) |
| 2c | Lemon | 47 | 210 | ― | 57.27(57.80) | 2.95(3.18) | 6.36(5.97) | 14.54(14.89) |
| 2d | Cream | 33 | 270 | ― | 64.54(64.08) | 3.91(4.05) | 3.42(3.66) | 15.64(15.16) |
| 2e | Lemon | 34 | 160 | 409(397.0) | 64.54(64.83) | 3.91(4.28) | 3.42(3.86) | 15.64(15.99) |
| 2f | Pich | 48 | 191 | ― | 64.54(64.23) | 3.91(3.46) | 3.42(3.22) | 15.64(15.60) |
| 2g | Cream | 46 | 219 | ― | 62.11(61.85) | 3.76(4.04) | 3.29(3.15) | 15.05(15.39) |
| 2h | Light yellow | 56 | 185 | ― | 58.67(58.12) | 3.02(3.26) | 3.25(3.17) | 14.90(15.36) |
| 2i | Yellow | 48 | 229 | ― | 63.79(63.79) | 3.54(3.98) | 3.54(3.58) | 16.20(15.71) |
| 3a | Cream | 18 | 147 | 456.5(441.1) | 55.20(55.90) | 2.84(3.24) | 6.13(6.30) | 14.01(13.74) |
| 3b | Yellow | 32 | 140 | 456.5(467.0) | 55.20(55.44) | 2.84(3.04) | 6.13(6.36) | 14.01(13.81) |
| 3c | Light yellow | 36 | 175 | 456.5(467.0) | 55.20(55.34) | 2.84(2.92) | 6.13(6.45) | 14.01(14.21) |
| 3d | Light lemon | 32 | 278 | ― | 62.04(61.96) | 3.76(4.06) | 3.29(3.59) | 15.04(15.04) |
| 3e | Pich | 42 | 157 | 425.5(417.8) | 62.04(61.83) | 3.76(4.01) | 3.29(3.06) | 15.04(14.87) |
| 3f | Cream | 48 | 193 | ― | 62.04(61.83) | 3.76(3.51) | 3.29(3.56) | 15.04(15.00) |
| 3g | Skin | 36 | 126 | 441.5(441.1) | 59.79(59.59) | 3.62(3.86) | 3.17(2.96) | 14.49(14.69) |
| 3h | Light yellow | 53 | 196 | ― | 56.50(56.74) | 2.91(2.70) | 3.13(3.28) | 14.34(14.00) |
| 3i | Lemon | 42 | 208 | ― | 61.24(61.54) | 3.40(3.69) | 3.40(3.62) | 15.55(15.77) |
| 4a | Skin | 28 | 206 | ― | 50.29(50.90) | 2.59(2.69) | 5.58(5.23) | 12.77(12.85) |
| 4b | Light yellow | 38 | 163 | 501(496.3) | 50.29(50.89) | 2.59(2.40) | 5.58(5.85) | 12.77(12.39) |
| 4c | Light brown | 53 | 144 | 501(496.3) | 50.29(50.72) | 2.59(2.52) | 5.58(5.33) | 12.77(12.26) |
| 4d | Brown | 21 | 236 | ― | 56.17(56.92) | 3.40(3.27) | 2.97(3.21) | 13.61(13.09) |
| 4e | Skin | 29 | 97 | 470(467.0) | 56.17(55.70) | 3.40(3.68) | 2.97(3.10) | 13.61(13.00) |
| 4f | Yellow | 32 | 152 | 470(467.0) | 56.17(56.71) | 3.40(3.70) | 2.97(3.28) | 13.61(14.08) |
| 4g | Yellow | 42 | 143 | 486(496.3) | 54.32(54.82) | 3.29(3.35) | 2.88(3.11) | 13.16(12.85) |
| 4h | Bright lemon | 34 | 187 | ― | 51.37(51.62) | 2.65(2.67) | 2.85(2.78) | 13.04(13.52) |
| 4i | Lightbrown | 29 | 219 | ― | 55.26(54.71) | 3.07(3.18) | 3.07(3.03) | 14.03(14.60) |
Table 2- Antimicrobial activity of compounds (MIC, µg/ml)
|
Compound |
Microorganisms |
|||||
| S.Subtilis | S.Aureus | E.Coli | P.Aeruginosa | C.Albicans | A .Niger | |
| 2e | 50 | 50 | 50 | 50 | 25 | 50 |
| 2g | 25 | 25 | 25 | 50 | 25 | 25 |
| 3a | 50 | 50 | 50 | 50 | 50 | 25 |
| 3e | 50 | 50 | 50 | 50 | 25 | 25 |
| 3i | 50 | ― | 50 | ― | 50 | 50 |
| 4a | 50 | 50 | 50 | 50 | 25 | 50 |
| 4g | 100 | 50 | 50 | 50 | 25 | 25 |
| Ampicillin | 64 | 100 | 64 | 100 | ― | ― |
| Griseofulvin | ― | ― | ― | ― | 80 | 80 |
References
- Kucukguzel, S. G., Orul, E. E., Rollas, S., Salin, F., Ozbek, A. Eur. J. Med. Chem., 37 :197 (2002).
- Rawal, R. K., Prabhakar, Y. S., Katti, S. B., Deelereq, E. Bioorg. Med. Chem., 13 : 6771 (2005).
- Singh, G. S., Molotsi, B. J Farmaco., 60 : 727 (2005).
- Rahman, V. P. M., Mukhtar, S. H., Ansari, W., Lemiere, G. Eur. J. Med. Chem., 40 : 173 (2005).
- Salunke, M. H., Filmwala, Z. A., Kamble, A. D. Orient. J. Chem., 27 : 1243 (2011).
- Desta, G., Abi, T., Upadhyay, R. K., Aman, D. Orient. J. Chem., 28 : 1791 (2012).
- Vigorita, M. G., Ottana, R., Monforte, F., Maccari, R., Trovato, A., Monforte, M. T., Taviano, M. F. Bioorg. Med. Chem. Lett., 11 : 2791 (2001).
- Kavitha, C. V., Basappa, S., Nanjunda, S., Mantelingu, K., Doreswamy, S., Sridhar, M. A., Prasad, J. S., Rangappa, K. S. Bioorg. Med. Chem., 14 : 2290 (2006).
- Ottana, R., Maccari, R., Barreca, M. L., Bruno, G., Rotondo, A., Rossi, A., Chiricosta, G., Di Paola, R., Sautebin, L., Cuzzocrea, S., Vigorita, M. G. Bioorg. Med. Chem., 13 : 4243 (2005).
- Kucukguzel, S. G., Kocatepe, A., Deelereq, E., Sahin, F., Gulluce, M. Eur. J. Med. Chem., 41 : 353 (2006).
- Shashikant, R., Prajact, K., Nachiket, S. D., Sunil, A. N., Deepak, S. M. Smita, K. P., Daithankar, A. V. J. Chem. And Pharm. Res., 1 : 191(2009).
- Sharma, S. C. Bull. Chem. Soc. Japan, 40: 2422(1967).
- Upadhyay, R. K., Agarwal, N., Gupta, N. J. Indian Chem. Soc., 70 ; 537 (1993).
- Upadhyay, R. K., Agarwal, N., Mishra, G. J. Indian Chem. Soc., 72 ;849 (1995).
- Vats, V., Upadhyay, R. K., Sharma, P. E-Journal of Chem., 73 : 1040 (2010).
- Raghav, G., Upadhyay, S., Upadhyay, R. K. Orient. J. Chem., Communicated (2013).
- Meyers, R. A. Wiley and Sons, O. J. Interpretation of Inferared Spectra, Ltd., A Practical Approach, 10815-10837 (2000).
- Hore, P. j. Oxford University Press, USA; Nuclear Magnetic Resonance, 1st edition (1995).

This work is licensed under a Creative Commons Attribution 4.0 International License.









