Study of Antioxidant Activity of Juglans regia leaves
Farhad Hatamjafari and Sanaz Abdi Goorabi*
Department of Chemistry, Faculty of Science, Islamic Azad University-Tonekabon Branch, Tonekabon, Iran.
Article Received on :
Article Accepted on :
Article Published : 10 Jun 2013
This study was designed to investigate the antioxidant activity for Juglans regia leaves. The Methanol extract from Iranian Juglans regia leaves that grow in Karaj and Tonekabon regions was examined. In addition, total amount of DPPH (1,1-diphenyl-2-picryl hydrazyl) radical scavenging activities and reductive power of crude extracted its different fractions were determined. The antioxidant activity data by DPPH has been compared in both regions.
KEYWORDS:Antioxidant activity; Juglans Regia; DPPH
Download this article as:| Copy the following to cite this article: Hatamjafari F, Goorabi S. A. Study of Antioxidant Activity of Juglans regia leaves. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Hatamjafari F, Goorabi S. A. Study of Antioxidant Activity of Juglans regia leaves. Available from: http://www.orientjchem.org/?p=11995 |
Introduction
Free radicals are linked with the majority of diseases and the partially reduced metabolites of oxygen and nitrogen, are highly toxic and reactive1-2. Oxidation is one of the most important route for producing free radicals in food, drugs and living systems. Antioxidants are the substances that when present in low concentration significantly delay or reduce the oxidation of the substrate. Antioxidants protect the living systems from damaging oxidation reactions by reacting with free radicals3. Juglans regia comprises many species and is widely distributed throughout the world. Its skins, seeds, barks, and leaves are widely used in the pharmaceutical and cosmetic industries4-5. Juglans regia leaves, which are easily available in large quantities can be considered as a source of compounds for health care, and in traditional medicine for the treatment of venous insufficiency, hemorrhoids, hypoglycemia, diarrhea, and fungal or bacterial infections6.
Natural compounds such as phenolic compounds and antioxidants have been shown to play a role in human health, including reducing the risk of degenerative diseases, oxidative stress, and inhibition of macromolecular oxidation7. The antimicrobial activity of the Juglans regia leaves has not been studied so far8. In addition several studies have shown that the antimicrobial activity of phenols and phenolic extracts and their excellent alternative to antibiotics and chemical preservatives9-11.
The present study aimed to evaluate the antioxidant (DPPH) propertyof the Juglans regia leaves collected from Tonekabon and Karaj regions in Iran.
Materials and Methods
Plant material and Extraction
Juglans regia leaves were collected in the spring of 2012 in Karaj city and Tonekabon city, Iran, from the branches exposed to sunlight. Juglans regia leaves were cut into small pieces and dried in the shade (22 °C) for 48-72 h. Dried leaves were ground into a fine powder using a homogenizer. A hydroalcoholic extract of the leaves was prepared by the maceration method, using a ratio of 1 g of Juglans regia leaves to 5 mL of MeOH (99%) for 48 h. Flasks were shaken several times. The extract was filtered and the solvent was evaporated using a rotary evaporator. The extract was then dried at 40 °C and powdered.
Antioxidant activity DPPH assay
The hydrogen atoms or electrons donation ability of the corresponding extracts and some pure compounds were measured by bleaching the purple colored methanolic solution of DPPH. The effects of methanolic extract and essential oil on DPPH radicals were evaluated according to a method described elsewhere. 4 mL samples of various concentrations of the extracts in methanol were separately added to a 1 mL solution of DPPH radical in methanol (final concentration of DPPH was 0.2 mM). The mixture was shaken vigorously and allowed to stand for 30 min after which the absorbance of the resulting solution was measured at 517 nm with a spectrophotometer (Rayleigh UV-1601). Inhibition of free radical DPPH as percentage [I(%)] was calculated as follows:
I (%) = 100 × (A blank -A sample) / A blank
Where Ablank is the absorbance of the control (containing all reagents except the test compound) and A sample is the absorbance of the test compound. IC50 value (μg /mL) is the effective concentration at which DPPH radicals are scavenged by 50%. This was obtained by interpolation and using linear regression analysis12.
DPPH determination
10 grams of powder was weighted and then mixed in 50 ml of methanol and then extracted for 24 hours in a lab temperature to get put, then the mixture was passed through the filter paper we put it in centrifuge for 20 minutes to obtain a concentrated extract (rpm 4000). After gaining weight again extracted with methanol extract (89%) prepare 100 ml the volume to make the mother solution. Mother solutions of different concentrations of extract, 100, 150, 200 and 250 mg/ml were used. To measure the antioxidant activity of the solution from Brand-Wiliames using DPPH, mg/ml were used12.
A small amount of the solution was poured into test tubes, each tube mother 10 ml of methanol added, Then 0.1 ml of each tube was removed and the other test tube, and it amounted to 3.9 ml DPPH solution were added. This was repeated three times, and 12 were measured for each concentration of test tubes 3 tube will. Test tubes were placed in the dark for 30 minutes if you have activity antioxidant change from purple to yellow color of antioxidant samples will be dissolved after its absorption at 517 nm by spectrophotometer was read.
Formula Antioxidant activity:
IC50 = 100 × (A-B / A)
Concentration of the extract, that take 50% of the trap free radicals = IC50
The absorbance of the control (DPPH and solvent-free extraction of all such factors) = A
The absorption of tested extract = B
It is seen in 0.5 ml of test solution +2.5 ml methanol solution of DPPH
Blank samples was methanol solution.
Table 1: Tables of Absorbance Concentrations of Extract, 100, 150, 200 and 250 Mg/Ml in Karaj Region
Test 1
|
No. |
Conc(mg/ml) |
Absorbance |
|
1 |
100 |
830 |
|
2 |
150 |
803 |
|
3 |
200 |
800 |
|
4 |
250 |
794 |
|
Blank |
|
866 |
Test 2
|
No. |
Conc(mg/ml) |
Absorbance |
|
1 |
100 |
566 |
|
2 |
150 |
539 |
|
3 |
200 |
439 |
|
4 |
250 |
436 |
|
Blank |
|
701 |
Test 3
|
No. |
Conc(mg/ml) |
Absorbance |
|
1 |
100 |
655 |
|
2 |
150 |
635 |
|
3 |
200 |
623 |
|
4 |
250 |
620 |
|
Blank |
|
701 |
Table 2: Tables of Absorbance Concentrations of Extract, 100, 150, 200 and 250 Mg/Ml in Tonekabon Region
Test 1
|
No. |
Conc(mg/ml) |
Absorbance |
|
1 |
100 |
631 |
|
2 |
150 |
366 |
|
3 |
200 |
203 |
|
4 |
250 |
81 |
|
Blank |
|
688 |
Test 2
|
No. |
Conc(mg/ml) |
Absorbance |
|
1 |
100 |
579 |
|
2 |
150 |
201 |
|
3 |
200 |
198 |
|
4 |
250 |
188 |
|
Blank |
|
701 |
Test 3
|
No. |
Conc(mg/ml) |
Absorbance |
|
1 |
100 |
497 |
|
2 |
150 |
246 |
|
3 |
200 |
227 |
|
4 |
250 |
201 |
|
Blank |
|
700 |
RESULTSANDDISCUSSION
Antioxidant DPPH assay results
To determine the antioxidant activity of the plant on Juglans regia, Tonekabon and Karaj regions with different concentrations were evaluated. In other words, in this experiment, both the pecan harvest (two levels) and concentration (4 levels) and the interaction of those factors was studied. Moreover, the analysis of variance(ANOVA) showed that different concentrations of the extract and concentration, as well as a significant effect on the interaction of free radicals had set a trap. We used the Duncan for treated groups to identify the concentration levels studied, multiple test. The result of this test is given in the following table.
Table 3: Duncan Test To Compare The Effect Of Concentration On The Antioxidant Activity Of The Juglans Regia
|
Standard Deviation |
Average |
Concentration |
|
32.6 |
61.21 |
100 |
|
77.14 |
51.34 |
150 |
|
12.12 |
3.43 |
200 |
|
30.12 |
21.44 |
250 |
The comparison of the results showed that the effect of the plant extract different concentrations on the trap free radicals weren’t up significantly. Graph below shows percentage trap of free radical of each of the concentration.
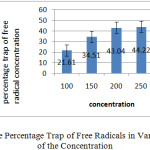 |
Graph 1: Percentage Trap of Free Radicals in Various of Each of the Concentration: Click here to View graph |
In the graph below, the percentage trap free radicals associated with each of these levels to distinguish Tonekabon and Karaj regions are shown.
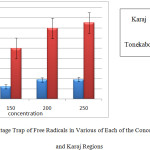 |
Graph 2: The Percentage Trap of Free Radicals in Various of Each of the Concentration for Tonekabon and Karaj Regions: |
As can be seen in the above chart, the percentage that trap free radicals Tonekabon region is much higher. This chart also shows that most caught up in the free radical concentration of the herbal extract Juglans regia Tonekabon region 250 mg/ml and the lowest is caught up in a concentration of 100 mg/ml herbal extracts. Different concentrations of plant extract, Karaj region in relation to trap free radicals have done the same. The following diagram interactions in plant samples and various concentrations of the plant extracts on the DPPH free radical trap shows.
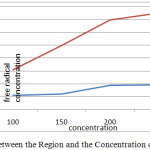 |
Graph 3: The Interaction between the Region and the Concentration of Free Radicals in the Trap: Click here to View graph |
This shows that most caught up in the free radical concentration is 250 mg/ml Tonekabon region and also set the lowest trap free radicals is 100 mg/ml for Karaj region.
Conclusion
The evaluation of the antioxidant activity by DPPH test has indicated that the extract of Juglans regia had accepteble antioxidant activity as this study showed most caught up in the free radical concentration was 250 mg/ml Tonekabon region and also set the lowest trap free radicals was 100 mg/ml for Karaj region.
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of Tonekabon Branch Islamic Azad University.
References
- Gutteridgde J., Free Rad. Res. Comm., 19: 141 (1995).
- Aruoma O., J. Am. Oil Chem. Soc., 75: 199 (1998).
- Halliwell B., Lancet., 355: 1179 (2000).
- Stampar F. , Solar A. and Hudina M., Food Chemistry., 95: 627(2006).
- Pereira J., Oliveira I. and Sousa A., Food. Chem. Toxicol., 45: 2287 (2007).
- Wichtl M. and Anton R., Plantes Thérapeutiques Technique et Documentation., Paris (1999).
- Qa’dan F., Thewaini A. and Ali D., Am. J. Chin. Med., 33: 197(2005).
- Oliveira I., Sousa A. and Ferreira I. , Food. Chem. Toxicol., 46: 2326(2008).
- Proestos C., Chorianopoulos N. and Nychas G., J. Agric. Food. Chem., 53: 1190(2005).
- Sousa A., Ferreira I. and Calhelha R., Bioorg. Med. Chem., 14: 8533 (2006).
- García-Cortés J., Medina-Solís C. and Loyola-Rodriguez J., Rev. Salud. Publica., 11: 82 (2009).
- Brand-Williams W., Cuvelier M. and Berset C., LWT-Food Sci Technol., 28: 25 (1995).

This work is licensed under a Creative Commons Attribution 4.0 International License.









