Spectral Study of Ruthinium (III) Catalyzed Oxidation of Maltose by Potassium Permagnate in Acidic Medium
Ashish Kumar, Zakir Gani and Sumayah Bashir
Chemical Laboratories, Lovely Professional University, Punjab, India.
Corresponding Author E-mail: drashishchemlpu@gmail.com
The kinetics of Ruthenium (III) catalyzed oxidation of maltose by potassium permagnate in acidic medium have been studied spectrophotometrically in present investigation. First order kinetics with respect to variation in KMnO4 concentration is observed. The order of reaction with respect to substrate is zero but with respect to Ru(III) is one in this reaction. Increase in [H+ ] shows positive effect, while increase in [Cl-] shows no effect. The active species of ruthenium (III) is understood as [Ru(H2O)4O]2+ . The reaction constants involved in the different steps of mechanism are calculated. Activation parameters with respect to the slow step of the mechanism are computed and discussed and thermodynamic quantities are also calculated. A suitable mechanism and rate law in conformity with the kinetic observations has been proposed in the end and product has been studied spectroscopically for confirmation.
KEYWORDS:Acidic medium; kinetics; oxidation; potassium permagnate; Ru(III)catalyzed; Maltose
Download this article as:| Copy the following to cite this article: Kumar A, Gani Z, Bashir S. Spectral Study of Ruthinium (III) Catalyzed Oxidation of Maltose by Potassium Permagnate in Acidic Medium. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Kumar A, Gani Z, Bashir S. Spectral Study of Ruthinium (III) Catalyzed Oxidation of Maltose by Potassium Permagnate in Acidic Medium. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22054 |
Introduction
Permanganates ions oxidize a greater variety of substrates and find extensive applications in organic syntheses,1–7 especially after the advent of phase transfer catalysis,3,4,6 which permits the use of solvents such as methylene chloride and benzene. Kinetic studies constitute an important source of mechanistic information on the reaction, as demonstrated by results referring to unsaturated acids in both aqueous1,3,7,14-17 and non-aqueous media.8. During oxidation by permanganate, it is evident that the Mn(VII) in permanganate is reduced to various oxidation states in acidic, alkaline and neutral media. Furthermore, the mechanism by which this multivalent oxidant oxidizes a substrate depends not only on the substrate but also on the medium9 used for the study. In strongly alkaline media, the stable reduction product10,11 Potassium permanganate has been used to oxidize various compounds in both acidic and alkaline medium [1-6]. Little attention has been paid however, to the reactivity of KMnO4 in the presence of catalyst [7,8] and nearly no investigation has so far been reported on the catalytic role of ruthenium(III) chloride with potassium permanganate as an oxidant in acidic medium. This fact prompted us to undertake the present investigation namely, “Mechanistic study of ruthenium(III) catalyzed oxidation of maltose by acidic potassium permanganate”.
Experimental
Devices
The change in absorbance with respect to time was done by Shimadzu UV 1800-Double beam-UV–Vis spectrophotometer and IR studies were performed by Shimadzu8400 S- FT IR,
Materials
An aqueous solution of Maltose (BDH), potassium permanganate (CDH, AR), KNO3 and were prepared by dissolving the weighed samples in triple distilled water. H2SO4 acid (SD.fine) was used as a source of H+ ion. The Ru(III) solution was prepared by dissolving a known weight of RuCl3 (SDFine-Chem) in HCl (0.20 mol dm–3). Hg was added to the Ru(III) solution, to reduce any Ru(IV) formed during the preparation of the Ru(III) stock solution, which was set aside for 24 h. The Ru(III) concentration was then assayed by EDTA titration.15.All other reagents were of analytical grade and their solutions were prepared by dissolving the requisite amounts of the samples in doubly distilled water. H2SO4 and KNO3 were used to provide the required acidity and to maintain the ionic strengthrespectively. Reaction vessels were painted black so as to prevent photochemical decomposition, if any.
Kinetics
All kinetic measurements were performed under pseudo-first order conditions with excess of [maltose] over [MnO4 – ] at a constant ionic strength of 0.30 mol dm–3. The reaction was initiated by mixing previously thermostated solutions of MnO4 – and maltose, which also contained the necessary quantities of Ru(III), H2SO4 and KNO3, to maintain the required acidity and ionic strength respectively. The temperature was uniformly maintained at 30 ± 0.1°C. The course of reaction was followed by monitoring the decrease in the absorbance of MnO4– in a 1 cm quartz cell of Shimadzu UV 1800-Double beam-UV–Vis spectrophotometer , at its absorption maximum of 525 nm as a function of time. The application of Beer’s law to permanganate at 525 nm is verified, giving Ɛ=2250±25dm3 mol–1 cm–1 (literature Ɛ = 2200 dm3 mol–1 cm–1). The first-order rate constants, were evaluated by the plots. The course of reaction was followed by recordinMnO4– at its absorption maximum, 525 nm, as a function of time9. It was verified that there was no interference from other reagents at this wavelength. The absorption spectra of the reactants and typical traces of the spectral changes are shown in fig A.
 |
Scheme 1 |
![Figure A: Spectroscopic changes occurring in the Ru(III) catalysed oxidation of maltose by permanganate with[S] = 1.0 X 10–3, [MnO4] = 1×5 ´ 10–4 [Ru(III)] = 1×10–6, [H+] = 1×10–3, and I = 0.30 mol dm–3 at 30°C, scanning time interval = 120 s.](http://www.orientjchem.org/wp-content/uploads/2013/06/Vol29No2_Spec_Ashi_figA-150x150.jpg) |
Figure A: Spectroscopic changes occurring in the Ru(III) catalysed oxidation of maltose by permanganate with[S] = 1.0 X 10–3, [MnO4] = 1×5 ´ 10–4 [Ru(III)] = 1×10–6, [H+] = 1×10–3, and I = 0.30 mol dm–3 at 30°C, scanning time interval = 120 s. |
Results and Discussion
The stoichiometry of the reaction was ascertained by equilibrating the reaction mixture containing an excess of potassium permanganate. Estimation of unconsumed MnO4– revealed that one mole of substrate consumed four moles of MnO4–
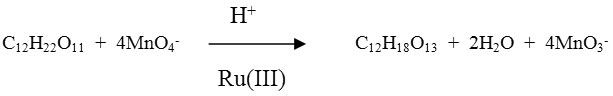
S = maltose P = Malturic acid
Ru(III) catalyzed oxidation of maltose was studied at several initial concentrations (Table-1). The plots of unconsumed manganate versus time on varying concentrations showed first order w.r.t. [MnO4–] which is further confirmed by plotting a graph between (-dc/dt) vs [MnO4–] (Fig.1.1). The first order rate constant shows nearly the same value at different concentrations of Maltose, showing zero order dependence on substrate concentrations. The reactions were observed to be first order with respect to RuIII. This has also been confirmed by “least- square method” (Fig.3). A log (-dc/dt) versus log [H+] plot (Fig.4) was found to have a slope value of +0.24 at 300 C, indicating a positive fractional order effect of [H+] on the reaction rate. Experimental data showed that the reaction was unaffected by chloride ion and ionic strength on the rate.
Table 1: Effect of variation of reactants on the reaction rates for maltose at 300C
|
[Substrate] x 102 M
|
[KMnO4] x 103 M |
Ru(III) x 106 M |
[HClO4] x 103 M |
(-dc/dt) x 107 mol l-1 s-1
|
|
0.33 0.40 0.50 0.66 1.00 2.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 |
1.00 1.00 1.00 1.00 1.00 1.00 0.28 0.33 0.40 0.50 0.66 1.00
1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 |
96 96 96 96 96 96 96 96 96 96 96 96 48 96 144 192 240 288
96 96 96 96 96
|
1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 0.33 0.40 0.50 0.66 1.00 2.00 |
8.47 8.65 8.45 8.55 8.59 8.34 2.23 2.8 3.21 4.15 5.35 8.59 4.8 8.59 11 16.2 20.33 24.21 5.83 6.05 6.84 7.34 8.59 9.50 |
[KNO3] = 0.30 M
![Figure 1.1: Plot between [KMnO4] x 10-3 M vs. (-dc/dt) x 10-7 M L-1 S-1 for oxidation of Maltose at 30°C.](http://www.orientjchem.org/wp-content/uploads/2013/06/Vol29No2_Spec_Ashi_fig1.1-150x150.jpg) |
Figure 1.1: Plot between [KMnO4] x 10-3 M vs. (-dc/dt) x 10-7 M L-1 S-1 for oxidation of Maltose at 30°C. |
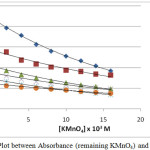 |
Figure 1.2: Plot between Absorbance (remaining KMnO4) and time at 30°C |
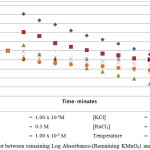 |
Figure 1.3: Plot between remaining Log Absorbance-(Remaining KMnO4) and time at 300C |
![Figure 2: Plot between [Rh(III)] x 10-5 M vs. (-dc/dt) x 10-7 M L-1 S-1 for oxidation of Maltose at 30°C.](http://www.orientjchem.org/wp-content/uploads/2013/06/Vol29No2_Spec_Ashi_fig2-150x150.jpg) |
Figure 2: Plot between [Rh(III)] x 10-5 M vs. (-dc/dt) x 10-7 M L-1 S-1 for oxidation of Maltose at 30°C. |
![Figure 3: Plot between log [Rh(III)] vs. log (-dc/dt) for oxidation of Maltose at 300C.](http://www.orientjchem.org/wp-content/uploads/2013/06/Vol29No2_Spec_Ashi_fig3-150x150.jpg) |
Figure 3: Plot between log [Rh(III)] vs. log (-dc/dt) for oxidation of Maltose at 300C. |
![Figure 4: Plot between 4 + log [H+] vs. 8 + log (-dc/dt) for oxidation of Maltose at 300C.](http://www.orientjchem.org/wp-content/uploads/2013/06/Vol29No2_Spec_Ashi_fig4-150x150.jpg) |
Figure 4: Plot between 4 + log [H+] vs. 8 + log (-dc/dt) for oxidation of Maltose at 300C.
|
The rate measurements were taken at 300 – 450C and a plot of log k vs I/T, which was linear (fig.5). In the light of the discussion regarding the reactive species of potassium permagnate in acidic medium and that of ruthenium trichloride in acidic medium it has been concluded beyond doubt that MnO4– and [RuCl2(H2O)4]+ are the actual reactive species of potassium permagnate and ruthenium trichloride respectively in acidic medium.Now on the basis of above results following mechanism can be given for the oxidation of aforesaid substrates:
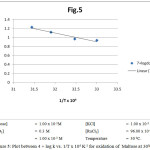 |
Figure 5: Plot between 4 + log k vs. 1/T x 104 K-1 for oxidation of Maltose at 300C. |
Considering the above slow steps and applying steady state treatment with a reasonable approximation, the rate law for Maltose may be written in terms of consumption of [MnO4–] as equation:-
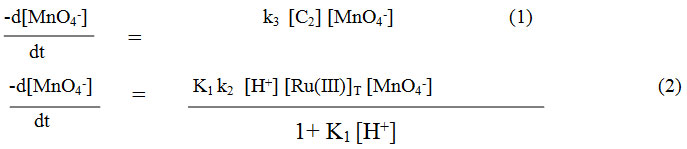
where K1 = (k1/k-1) and [Ru(III)]T = [C1] + [C2]
Spectral Evidence for the Formation of Complexes During Course of Reaction
In the oxidation of maltose with permanganate ion in acidic medium, order with respect to sugars concentration was first throughout its 10-fold variation. This shows that OH group of sugar involves in the oxidation before the rate determining step although it will combine with the most reactive complex to form the reaction products along with the regeneration of Ru catalyst .In order to probe the possible formation of complex MnO4 – and Ru (III) spectra for the solution of MnO4–, H+ and for the solution of MnO4– , H+ and Ru(III) have been collected Figures.
From the spectra, it is clear that the addition of Ru (III) solution there is a decrease of absorbance from 1.567 to 1.465 (λmax 525 nm). This decrease in absorbance can be considered as an indication of quick formation of the complex between reactive species of Ru (III) in acidic medium. When maltose and lactose solutions were added to the solutions of MnO4 – , H+ and Ru(III) a decrease in absorbance from 1.465 to 1.376 (λmax 525 nm)for maltose was noted (Fig B- Fig.D). This shows that the complex formed subsequently combines with maltose to give a highly solvated activated complex.
 |
Figure B: Absorption Spectra of MnO4 -, H+ solutions. |
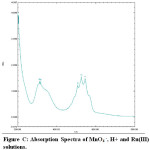 |
Figure C: Absorption Spectra of MnO4 –, H+ and Ru(III) solutions. Click here to View figure |
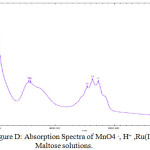 |
Figure D: Absorption Spectra of MnO4 –, H+ ,Ru(III) and Maltose solutions. |
Thus rate law (2) derived fully explains first order dependence of the reaction on ruthenium(III) chloride, zero order dependence of substrates viz. maltose in acidic medium. It also explains first order kinetics with respect to [MnO4–]
The rate law (2) also shows positive fractional order dependence on sulphuric acid i.e. [H+] and negligible effect of potassium chloride is shown.There is negligible effect of ionic strength and Acetic acid on reaction.
The rate expression shown by equation (2) can also be written as:-
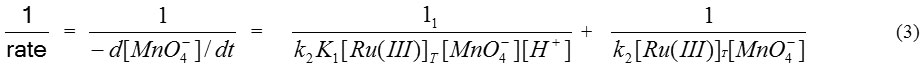
where K1 = (k1 / k-1)
With the help of equation (3) a graph plotted between (1/rate) and (1/[H+]), a straight line with positive intercept on Y axis (Fig. is obtained which helps us to calculate value of k2 and slope of straight line helps to calculate K1.
The rate law is in agreement with all observed kinetics. The proposed mechanism is in consistency with the activation parameters given in table-2.
Table 2: Activation parameters for acidic permanganate oxidation of Maltose
| Substrate | Temperature coefficient | ΔEakJ mol-1 | ΔH*J mol-1 | ΔS*JK mol-1 | ΔG*kJ mol-1 | Log A | krmol-2 lit-2 sec-1 |
| Maltose | 2.09 | 37.39 | 37.3947 | -214.04 | 64.879 | 1.809 | 63.64 |
Conclusions
In the present investigation it has been tried to experimentally explore various aspects of kinetics of oxidation of Maltose using Ruthenium (III) as a catalyst using potassium permagnate in acidic solution. The experimental results as shown reveal that the reaction rate doubles when the concentration of the catalyst Ruthenium (III) is doubled. The order w.r.t. potassium permagnate is also observed to first order. There is fractional order w.r.t. H+ in reaction mixture. The rate law is in conformity with all kinetics observations summarized there. The product is Malturic acid and is confirmed by TLC, Functional Group Analysis and also by FTIR Spectroscopy. The proposed mechanistic steps are supported by the negligible effect of ionic strength which indicates the involvement of an ion in the slow and rate determining step. From the present investigation it is also concluded that MnO4– and [RuCl2(H2O)4]+ are the reactive species of KMnO4 and Ruthenium(III) chloride respectively in acidic medium.The high positive values of energy of activation ,(∆G⃰ ) indicates highly solvated transition state, while fairly high negative values of entropy of activation (∆S⃰ ) suggest the formation of an activated complex with reduction in degree of freedom.
References
- Sh. Srivastava , R.K. Sharma ; Oxidation Communication , 2, 343-349 (2006).
- Hassan R M, Mousa M A and Wahdan M H.; 1988., J Chem Soc, Dalton Trans, 605-609(1988.)
- Sheila Srivastava and Sarika Singh ; J. Indian Chem. Soc., 18 , 295 (2004).
- Sheila Srivastava , Ashish Kumar and Parul Srivastava ; J. Indian Chem. Soc., 83 , 347-350 (2006).
- Sheila Srivastava , Rajendra Kumar Sharma and Sarika Singh ; J. Indian Chem. Soc., 83 , 282-287 (2006).
- Ashish Singh , Surya Singh , Ashok Singh , Bharat Singh ; Trans. Metal Chem. , 30(5) , 610-615 (2005).
- Iftikhar Ahmed , C. Mohammad Ashraf ; Int. J. Chem. Kinet. , 11 , 813-819 (2004).
- Saik Sondu , Bangalore , Sethuram and Tageda Navaneeth Rao ; Transition Metal Chem. , 15(1) , 78-80 (1990).
- Sheila Srivastava ; Trans. Metal Chem. , 24(6) , 683-685 (1999).
- Sheila Srivastava , Bharat Singh ; Trans. Metal Chem., 16 ,466 (1991).
- N. Venkata Subramanian , V. Thigarajan ; Canadian J. Chem., 47 , 694 (1969).
- J. C. Bailer , “ Chemistry of Coordination Compound ; Reinhold , New York (1964) , P-4.
- C. S. Reddy , E. V. Sundaram ; J. Indian Chem. Soc., 62 , 209 (1985).
- Sheila Srivastava , Ashish Kumar and Parul Srivastava ; Oxidation communications, 29 , No. 3,660-666 (2006).
- Reddy C S and Vijayakumar T 1995 Indian J. Chem. A34 615; 1970 Chem. Abstr. 72 50624
- Sheila Srivastava , Ashish Kumar and Parul Srivastava ; Oxidation communications, 29 , No. 3,622-627 (2006).
- Sheila Srivastava , Ashish Kumar, Parul Srivastava, Lakshmi chaudhary and Shalini singh, J. Indian Chem. Soc., 86 , 1-5 (2009).

This work is licensed under a Creative Commons Attribution 4.0 International License.









