Reduction of α-Diketones and Acyloins with Zn(BH4)2/ZrCl4 to their Corresponding Vicinal Diols
Rasol Kamari and Davood Setamdideh*
Department of Chemistry, Faculty of Sciences, Mahabad Branch, Islamic Azad University, Mahabad, 59135-443, Iran.
Correspondig Author E-mail: davood.setamdideh@gmail.com
α-diketones and acyloins are reduced to the corresponding vicinal diols with Zn(BH4)2/ZrCl4system in THF at room temperature.
KEYWORDS:α-Diketones; Acyloins; Zn(BH4)4; ZrCl4
Download this article as:| Copy the following to cite this article: Kamari R, Setamdideh D. Reduction of α-Diketones and Acyloins with Zn(BH4)2/ZrCl4 to their Corresponding Vicinal Diols. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Kamari R, Setamdideh D. Reduction of α-Diketones and Acyloins with Zn(BH4)2/ZrCl4 to their Corresponding Vicinal Diols. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22138 |
Introduction
Reduction ofα-hydroxy ketones and α-diketones to vicinal diols and/or acyloins are the subject of interests in organic synthesis1. Reduction of α-diketones usually gives a mixture of α-hydroxy ketonesand vicinal diols. On the other hand, using of some reagents (chemical or biochemical) can undergo selective reduction of α-diketones to only α-hydroxy ketones or vicinal diols2-6.However, Cryptococcus macerans7, modified tetra-hydroborate agents 8a, NaBH4/DOWEX1-X88b system and NaBH4/DOWEXRWX48chave been reduced α-diketonesand acyloins to vicinal diols. In addition, reduction of acyloins to vicinal diolshas been achieved by usingH2/CuCr2O4atroom temperature9.
Results and Discussions
Recently, we have demonstrated that Zn(BH4)2 is a sufficient reducing agent for the reduction of carbonyl compounds under different combination systems such as Zn(BH4)2/H2O10, Zn(BH4)2/C11, Zn(BH4)2/Al2O312and Zn(BH4)2/2NaCl13. So, In this context and in continuing our efforts for the development of new reducing systems 10-14, we wish to introduce Zn(BH4)2/ZrCl4 as a new combination reducing system for fast and efficient reduction of acyloins and α-diketones to their corresponding vicinal diols. The model reaction has been selected by reduction of benzil to 1,2-diphenylethane-1,2-diol. This reaction was carried out in different solvents, different molar ratio of the Zn(BH4)2/ZrCl4for the selection of appropriate conditions at room temperature. Among the tested different solvents, the reaction was most facile and proceeded to give the highest yield in THF. The optimization reaction conditions showed that using 2 molar equivalents of Zn(BH4)2 and 0.4 molar equivalents of ZrCl4in THFwas the best conditions to complete the reductionof benzil (1 mmol). Our observation reveals that reduction reaction completes within 40 min with 92% yields of product as shown in scheme 1.
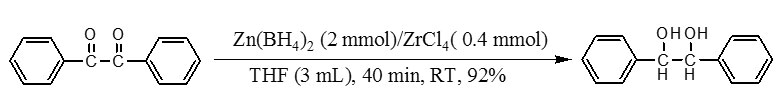
This procedure was also applied for the reduction of various α-diketones tothe corresponding vicinal diols(Table 1, entries 1-4). All reductions were completed within 40-50 min by 2 molar equivalents of Zn(BH4)2and 0.4 molar equivalents of ZrCl4in excellent yields of products(92-95%).Our next attempt was the reduction of α-hydroxy ketones to the corresponding vicinal diols. The reduction of acyloins to their corresponding vicinal diols (94-96%) was also obtained successfully by 2 molar equivalents of Zn(BH4)2 in the presence of 0.4molar equivalents of ZrCl4within 50-60 minat room temperature in THF (Table 1, entries 5-7).Our attempts for reduction of α-diketones to acyloins were unsatisfactory and only vicinal diols were identified as thesole products.
Experimental
IR and 1H NMR spectra were recorded on PerkinElmer FT-IR RXI and 400 MHz Bruker spectrometers, respectively. The products were characterized by their 1H NMR or IR spectra and comparison with authentic samples (melting or boiling points). TLC was applied for the purity determination of substrates, products and reaction monitoring over silica gel 60 F254 aluminum sheet.
Typical Procedure for the Reduction of α-Diketones and Acyloins with Zn(BH4)2/ZrCl4 System in THF
In a round-bottomed flask (10 mL) equipped with a magnetic stirrer bar, a solution of benzil (0.21 g, l mmol) was prepared in THF (3 mL). To this solution, ZrCl4 (0.4 mmol, 0.93 g) and Zn(BH4)2 (0.19 g, 2mmol) was added. The resulting mixture was stirred at room temperature for 40 min. The reaction was monitored by TLC (eluent:CCl4/Et2O:5/2). After completion of the reaction, distilled water (6 mL) was added to the reaction mixture and stirred for 5 min. The mixture was extracted with CH2Cl2 (3×10 mL) and dried over anhydrous Na2SO4. Evaporation of the solvent afforded crystals of 1,2-diphenyl ethane-1,2-diol (0.19 g, 92% yield).
Conclusion
In this context, we have shown that the Zn(BH4)2/ZrCl4 as new reducing system is convenient for the reduction of α-hydroxy ketones and α-diketones to their corresponding vicinal diols. Reduction reactions were carried out with Zn(BH4)2(2mmol) and ZrCl4(0.4mmol) in THF at room temperature. Short reaction times, low reaction temperature and easy work-up procedure makes as an attractive new protocol for the reduction of α-diketones and acyloinsto their corresponding vicinal diols, therefore it could be a useful addition to the present methodologies.
Acknowledgements
The authors gratefully appreciated the financial support of this, work by the research council of Islamic Azad University branch of Mahabad.
References
- March, J. Advanced Organic Chemistry, 4th Ed.; John Wiley &Sons: NY (1992). (b) Greene, T. W.; Wuts, P. G. M. Protective Groups in Organic Synthesis; John Wiley & Sons: NY, (1999) (c)Matsuda, F.; Kawasaki, M.; Terashima, S. Tetrahedron Lett.,26: 4639 (1985) (d) Guiso, M.; Procaccio, C.; Fizzano, M. R.; Piccioni,F. Tetrahedron Lett.,38: 4291(1997).
- Pechmann, V. H.; Dahl, F. Chem. Ber., 23: 2421 (1890).
- Ho, T.L.; Olah, G. A. Synthesis,815 (1976).
- Mori, T.; Nakahara, T.; Nozaki, H. Can. J. Chem.,47: 3266 (1969).
- Mayer, R.; Hiller, G.; Nitzschke, M.; Jentzsch, J. Angew. Chem.,75: 1011 (1963).
- Rubin, M. B.; Ben-Bassat, J. M. Tetrahedron Lett.,12: 3403 (1971).
- Imuta, M.; Ziffer, H. J. Org. Chem.,43: 3530 (1978).
- Firouzabadi, H.; Zeynizadeh, B. Bull. Chem. Soc. Jpn.,70: 155 (1997). b)Zeynizadeh, B.; Shirini, F. Bull. Korean Chem. Soc.24: 295 (2003). c) Setamdideh, D.: Karimi, Z. and Alipouramjad, A. J. Chin. Chem. Soc., doi: 10.1002/jccs.20130014.
- Blomquist, A. T.; Goldstein, A. Org. Synth. Coll. Vol. IV: 216 (1963).
- Setamdideh, D.;Khezri, B.;Rahmatollahzadeh, M.;Aliporamjad, A.Asian J. Chem. 24:3591(2012).
- Setamdideh, D.;Rahmatollahzadeh, M.J. Mex. Chem. Soc.,56:169 (2012).
- Setamdideh, D.;Khezri, B.;Rahmatollahzadeh, M.J. Serb. Chem. Soc.,79:1 (2013).
- Setamdideh, D.;Khaledi, L. S. Afr. J. Chem. (2013), in press.
- Mohamadi, M.;Setamdideh, D.;Khezri, B.Org. Chem. Inter., doi:10.1155/2013/127585 (2013).

This work is licensed under a Creative Commons Attribution 4.0 International License.









