Oxidation of O-toluic Acid Hydrazide by Thallium (III) in Acidic Medium (A Kinetic and Mechanistic Study)
Yashodhara Varale1 and Amit Varale*
*Department of Chemistry, Athalye, Sapre, Pitre College, Devrukh (Ratnagiri).
1Department of EVS, Dr.Ambedkar College of Commerce and Economics, Wadala, Mumbai-31, India.
Correspondinga Author E-mail: amitvarale@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/290239
The kinetics of oxidation of o-toluic acid hydrazide by Thallium (III) in a mixture of perchloric and hydrochloric acid media at a constant ionic strength has been planned to study iodometrically. The reaction proceeds through formation of complex with reactant, which decomposes in subsequent steps to give product. Effect of acrylonitrile shows, that there is no formation of free radicals. The increase in [H+] and [Cl-] decreases the rate of the reaction. The increase in ionic strength does not affect the rate of reaction. The effect of temperature was studied at four different temperatures ranging from 150C to 300C. The activation parameters were also determined and a mechanism is predicted.
KEYWORDS:o-toluic acid hydrazide; kinetics; thallium (III); oxidation
Download this article as:| Copy the following to cite this article: Varale Y, Varale A. Oxidation of O-toluic Acid Hydrazide by Thallium (III) in Acidic Medium (A Kinetic and Mechanistic Study). Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Varale Y, Varale A. Oxidation of O-toluic Acid Hydrazide by Thallium (III) in Acidic Medium (A Kinetic and Mechanistic Study). Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22205 |
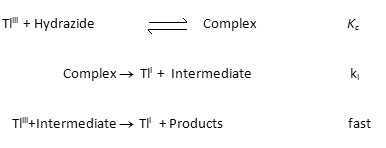
Introduction
Thallium oxide is one of the versatile available oxidizing agents used for oxidation of hydrazide. The reaction of hydrazide with most oxidants give the corresponding acids1 and in some cases2 esters or amides. Thallium(III) salts are well known oxidants3 in organic synthesis. Hydrazides are pharmaceutically important compounds used as antitubercular4 and antibacterial5,6 agents, some of them have been reported to possess anti-inflammatory7 and diuretic8 activities. The redox potential of Tl(III)/Tl(I) couple is sensitive to the anion present in the solution. In perchloric and sulphuric acid media9 it has the highest value of 1.23V with eitherfreeTl3+,TlOH2+ or thallium(III) sulphate complexes as active species respectively. Therefore, thallium(III) can be utilized both as a strong (in perchloric acid and sulfuric acid media) and as a mild oxidant10 (in hydrochloric acid medium) by changing the reactive species.
Material and Methods
Thallium (III) solution was prepared by dissolving Tl2O3 (ACROS) in 1.0 moldm-3 HCl and the concentration was ascertained by iodometric titration. The o-toluic acid hydrazide was prepared from reported[11] procedure and characterized by determining its melting point. Stock solution of o-toluic acid hydrazide was prepared in 50 % v/v, 1,4-dioxane. Ionic strength was kept constant.
The reactions were carried out in 50 % v/v 1-4 dioxane (s.d.fine.chem) under pseudo first order conditions keeping concentration of hydrazide in large excess over that of the oxidant. The solutions containing the reactants and all other constituents were thermally equilibrated separately, mixed and the reaction mixture was analysed for unreacted thallium (III) iodometrically by titrating against standard thiosulphate. The pseudo-first order rate constants were determined from the slopes of linear log[Tl(III)] versus time plots. The results were reproducible up to ± 5 %. Kinetic runs were followed to about three half-lives of the reactions. Under the experimental condition oxidation of 1,4-dioxane did not occur.
The stoichiometry of the reaction was determined using a known excess of thallium (III) over hydrazide and determining remaining oxidant iodometrically after 24 hrs. The results consistent with equation-(1) were obtained. The corresponding o-toluic acid was characterized by determining its MP.
![]()
End product analysis. For identification of products the reaction was carried out by using aqueous solution of hydrazide, thallium(III), HCl and HClO4. The flask containing reaction mixture was kept in thermostated water bath maintained at 50oC for 24 h to complete the reaction, the residue obtained after filtration was analysed for acid as follows:
(i) The presence of o-toluic acid group was detected by testing with bicarbonate.
(ii) The formation of acid was confirmed by IR and its melting point.
Results
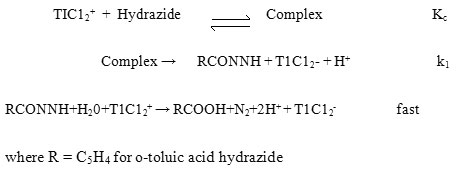
The reaction occurs rapidly in perchloric acid medium, but in the presence of hydrochloric acid the rate is measurable. Therefore, the reaction was carried out in a mixture of both the acids. The effect of reactants on the reaction was studied at constant [HCl] and [HClO4] of 0.1 mol dm–3 each and ionic strength of 0.6 mol dm–3. Concentration of oxidant was varied from 6.4 ×10-4 to 6.4 × 10-3 mol dm–3 keeping [hydrazide] constant at 1×10-1 mol dm–3. Since the pseudo-first order rate constants were fairly constant (3.60± 0.1 × 104 s-1 for m-TAH at 25oC) the order with respect to [oxidant] is unity. The effect of [hydrazide] was studied between the concentration range from 1×10-2 to 1×10-1 mol dm–3 keeping the [oxidant] constant at 3.0 ×10-3 mol dm–3. The pseudo-first order rate constants increase with increase in concentration and the order with respect to hydrazide is found to be fractional.
To study the effect of [H+] and [Cl–], [oxidant], [hydrazide] and ionic strength were kept as 3.0×10-3, 1×10-1 and 0.6 mol dm–3, respectively. To vary [H+] and [Cl–], HClO4 and NaCl were used. Increase in [H+] from 0.13 to 0.60×10-2 mol dm–3 decreases k ×10-4 (s-1) from 28.71 to 0.21 for m-TAH at 25oC. Increase in [Cl–] from 0.13 to 0.60 mol dm–3 decreases k ×10-4 (s-1) from 3.03 to 0.12 for m-TAH at 25oC. The relative permittivity was varied by changing the 1,4-dioxane content from 5 to 40% v/v. The rate was found to decrease with decrease in relative permittivity.
Added acrylonitrile in the concentration range from 0.5 to 2.5 vol.%, by keeping concentrations of oxidant, reductant, perchloric acid, hydrochloric acid and ionic strength fixed, did not produce any precipitate due to polymerisation of the added acrylonitrile, indicating absence of free radicals.
Disscussion
Since there is no formation of free radicals in the reaction, the reaction proceeds with two-electron transfer step.
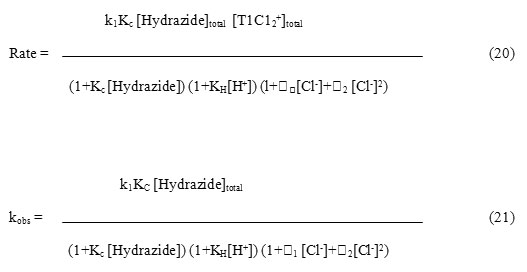
The order in thallium (III) was found to be unity and the order in hydrazide was found to be fractional. Such fractional order in substrate concentration is due to the prior complex formation equilibrium between the reactants. The Michealis – Menten plots of 1/kobs versus 1/[Hydrazide] were linear with an intercept in support of the complex formation. Therefore, in agreement with the results obtained the mechanism of the reaction can be represented as in Scheme 1. Equation 2 gives the rate according to Scheme1. Since, total [TlIII] exists in the form of free [TlIII] and the complex (Equation 3) therefore, the [TlIII] free is given by Equation 6. The overall rate law is now expressed by Equation 7 and the Pseudo-first order rate constant kobs, by Equation 8.

Rate law 8 is verified by plotting 1/kobs against 1/[Hydrazide] at four different temperatures and from the slopes and intercepts of these plots the values of k1 and Kc were calculated and are given in Table 1.
Table 1: Values of Kc and k1
| Temperature0C | KC (mol-1dm3)[o-TAH] | k1 X 104 (S-1)[o-TAH] |
| 15 | 14.00 | 2.38 |
| 20 | 09.52 | 5.00 |
| 25 | 09.23 | 8.33 |
| 30 | 07.05 | 16.66 |
The effect of hydrogen and chloride ion concentrations on the reaction is due to the protonation of hydrazides and different chloro – complexes of thallium (III) present in the solution. Hydrazides are known to be protonated in acid medium according to Equation 9.
![]()
Therefore, total [Hydrazide] can be expressed by Equation 10 and thereby the fact that there was no effect of free [Hydrazide] by Eq. 12. Since the rates of reaction decreases as the [H+] increases, free hydrazide is the active species. This is in support of ionic strength on the reactions indicating one of the reactant is neutral.
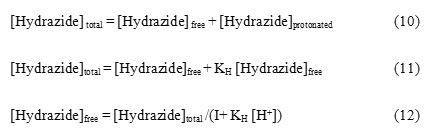
Thallium (III) forms strong complexes with chloride ions of the formula TlCln3-n where n is the number of chlorides complexes with thallium (III) as represented in equilibrium 13 to 16. The values of respective stability constants are K1 = 1.38 X 108, K2 = 3.98 X 1013, K3 = 6.02 X 1015 and K4 = 1.0 X 1018 mol–1dm3.
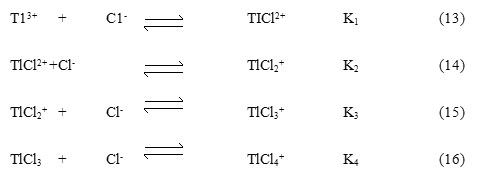
All the thallium(III) will exists as TlCl2+ and its concentration can be expressed by Equation 17. The [TlCl2]+free can now be given by eq. 19 where, b1 = K3/K2 = 151 and b2 = K4/K3 = 166, further, using Equations 18 and 19 the concentrations of [TlCl2]+free, TlCl3 and TlCl4– were calculated at different chloride ion concentrations and compared with the change in rate constant as the chloride ion concentration varied.
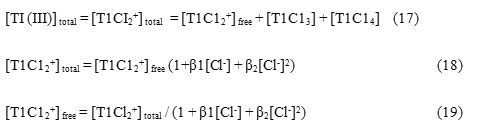
The concentration of both of [TlCl2+] free and TlCl3 parallel the values of rate constants as [Cl–] changes but the order [Cl–] is – 1.5, which makes [TlCl2+] free as the only active species.
where R = C5H4 for o-toluic acid hydrazide
Scheme 2
The mechanism considering TlCl2+ of oxidant and free hydrazide of the substrate as the active species can now be represented by scheme 2 with respective rate law and the expression for the pseudo-first order rate constants by Equations 20 and 21. The rate law 21 was verified by plotting 1/kobs against 1/[Hydrazide] and 1/kobs against [H+] which were found to be linear. From the slopes and intercepts of these plots the values of Kc and KH were determined.
The values of Kc are given in Table 1 and those of KH were found to be 13 and 16 mol-1 dm3 for o-toluic acid. The electrophilic character of TlCl2+ among the thallium (III) chlorocomplexes is highest thus making it the reactive species.
Michaelis-Menten plot for o-TAH
| Temperature | [o-t AH] | 1/[o-t AH] | kobs x 10-4 | 1/kobs x104 |
| 15 0C | 1×10-2 | 100.00 | 0.32 | 3.12 |
| 3×10-2 | 33.33 | 0.65 | 1.53 | |
| 5×10-2 | 20.00 | 0.98 | 1.02 | |
| 6.4×10-2 | 15.625 | 1.20 | 0.83 | |
| 10.0×10-2 | 10.00 | 1.96 | 0.51 | |
| 20 0C | 1×10-2 | 100.00 | 0.48 | 2.08 |
| 3×10-2 | 33.33 | 1.10 | 0.90 | |
| 5×10-2 | 20.00 | 1.60 | 0.62 | |
| 6.4×10-2 | 15.625 | 2.10 | 0.47 | |
| 10.0×10-2 | 10.00 | 2.90 | 0.34 | |
| 25 0C | 1×10-2 | 100.00 | 0.70 | 1.42 |
| 3×10-2 | 33.33 | 1.65 | 0.60 | |
| 5×10-2 | 20.00 | 2.0 | 0.50 | |
| 6.4×10-2 | 15.625 | 3.03 | 0.33 | |
| 10.0×10-2 | 10.00 | 3.6 | 0.27 | |
| 30 0C | 1×10-2 | 100.00 | 1.10 | 0.90 |
| 3×10-2 | 33.33 | 2.94 | 0.34 | |
| 5×10-2 | 20.00 | 4.54 | 0.22 | |
| 6.4×10-2 | 15.625 | 5.55 | 0.18 | |
| 10.0×10-2 | 10.00 | 8.33 | 0.12 |
Michaelis-Menten plot for o-TAH
 |
Figure 1 Click here to View figure |
 |
Scheme 1 Click here to View scheme |
The detailed mechanism involves electrophilic substitution on the nitrogen of the hydrazide with the formation of N-Tl bond, which decomposes in the subsequent step with, direct two-electron transfer from hydrazide to thallium to give an intermediate followed by fast steps. (Scheme 3). Such N-T1 bond formation has been postulated during thallium (III) oxidation of nitrogen containing compounds.
The activation parameters, with respect to slow step, k1, D H# (KJ mol-1), DG# (KJ mol-1) and DS# (JK-1mol-1) were found to be 32.76, 107.04 and -249.26 respectively for o-toluic acid hydrazide. Considerable decrease in the entropy of activation is due to formation of more ordered transition state as shown in scheme 3. The mechanism involves neutral hydrazide as the active substrate thus the reaction is unaffected by the change in the ionic strength. The increase in 1,4- dioxane content in the reaction medium decreases; the rate such an effect of the solvent is due to the stabilization of the complex formed between reactants in a medium of low relative permittivity.
Acknowledgement
The authors are thankful to Dr.N.P.Tendulkar, Principal, Athalye, Sapre, Pitre College, Devrukh (Ratnagiri) for his keen interest and help during the course of work.
References
- Winterstein A. Hegedus H., Inhibition of [3H] GABA Binding to Postsynaptic Receptors in Human Cerebellar Synaptic Membranes by Carboxyl and Amino Derivatives of GABA.
- Helv. Chem. Acta., 39, 229-235 (1956).
- Madzhoyan A. L. Handbook of Chemistry, Arm. Khim. Zh., 19, 793-798 (1966).
- Mckillop A and Taylor E. C., Organic synthesis by oxidation with metal compounds, J. Am. Chem Soc., 90, 7064- 70 (1980).
- Radhakrishnamurthi P. S. and Patil S. N., Kinetics and Mechanism of Oxidation of Oximes. J. Chem., 17A, 97-101 (1979).
- Radhakrishnamurthi P. S., Patil S. N., Kinetics and Mechanism of Oxidation of Ketones, J. Chem., 16A, 139-142 (1978).
- Vogel A. I.; Textbook of Practical Organic Chemistry. 4th ed. ELBS & Longman Group 1125 (1975).
- Lee A. G., The Chemistry of Thallium. Elsevier C. London., 48(1971).
- Amis E. S. Solvent effects on reaction rates and mechanisms, Academic Pres., New York., (1966).
- Varale A. S. and Hilage N. P. Kinetics and Mechanism of Oxidation Reactions by Thallium (III) in Acidic Medium. Oxid. Commun.. 31, 537-545 (2008).
- Varale A. S., Hilag, N.P., Oxidation of p-toluic acid hydrazide by Thallium(III) in Acidic Medium, Asian J. of Chemistry, 21, 1265-1272 (2009).
- Varale A. S. and Hilage N. P., Comparative Kinetic and Mechanistic study of Oxidation of Benzoic and p– nitro Benzoic Acid Hydrazide By Thallium(III) in Acidic Medium. Oriental J. of Chemistry., 24, 545-549 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









