Kinetics and Mechanism of Oxidation of Malic Acid by N-bromoanisamide
L. N. Malviya1, V. K. Siriah2 and M. K. Badole2
1Department of Chemistry Government P. G. College Pipariya (M.P.) India
2Department of Chemistry Government M. G. M. P. G. College Itarsi (M.P.) India
Article Received on :
Article Accepted on :
Article Published : 10 Jun 2013
The kinetics of the oxidation of the malic and by N-bromoanisamide in HC1O4 and in the presence of Hg(OAc)2 have been studied. The reactions exhibit a first order rate dependence with respect to the oxidant and substrate. The reactions are acid catalyzed and retarded by the addition of anisamide, a by product of reaction. The rate of oxidation decreases with decrease in dielectric constant of the medium. The effect of temperature on the reaction has been investigated in the temperature range 313-328 K. The stoichiometric studies revealed 1:1 mole ratio. Various thermodynamic parameters have been computed and a possible operative mechanism is proposed.
KEYWORDS:malic acid; oxidation; mechanism; N-bromoanisamide
Download this article as:| Copy the following to cite this article: Malviya L. N, Siriah V. K, Badole M. K. Kinetics and Mechanism of Oxidation of Malic Acid by N-bromoanisamide. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Malviya L. N, Siriah V. K, Badole M. K. Kinetics and Mechanism of Oxidation of Malic Acid by N-bromoanisamide. Available from: http://www.orientjchem.org/?p=11992 |
Introduction
Recently considerable attention has been focused on the diverse nature of the chemistry of N-halo compounds, due to their ability to act as sources of halonium cation, hypo halite species and nitrogen anion which act as base and nucleophiles. A review of literature shows that N-halo-compound such as N-bromsuccinimide1-4 N-bromacetamide5, N-bromobenzamide6, N-bromophthalimide7, N-chlorosaccharin8-9, N-halosulphonamide10-13(chloramine-T, bromamine-T, chloramine-B, bromamine-B etc) are commonly used for the oxidation of various organic compounds such as alcohols, aldehydes, amino acids, keto acids and hydroxy acids etc. N-bromoanisamide is also potential oxidant which has not been used so far, especially in oxidation of hydroxy acids. Many well known hydroxy acids are useful building blocks in organic synthesis, the most common and simple are malic acid, glycolic acid, lactic acid, acid, and mandelic acid etc. Malic acid is a dicarboxylic hydoxy acid which is contributes to the pleasantly sour taste of fruits and is used as food additive. Malic acid is widely used in medications, foods, industry.14
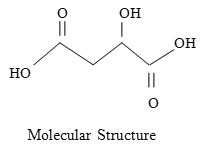
Experimental
N-bromoanisamide was prepared by the reported method and its purity checked by an iodometric method. All the other chemicals used were of AR grade. Solution of NaClO4 perchloric acid and mercuric acetate were prepared in doubly distilled water. Water (de-ionized) and glacial acetic acid were used as the solvent through the work. A thermostated water bath was used to maintain the desired temperature.
Results and Discussion
The kinetics of oxidation of malic acid is found pseudo first order. Reactions were initiated by addition of the NBA solution. The progress of reaction was followed by determining NBA iodometrically in aliquots withdrawn after suitable time. Stoichiometry of the reaction was studied and found that one mole of hydroxy acid consumes one mole of NBA in very case. The results may be represented by the following stoichiometric equation which indicate mole ratio 1:1.
![]()
Effect of N-bromoanisamide-
A plot of log [NBA] versus time gave a straight line which indicates that reaction under chosen condition follows pseudo first order kinetics. (table1)
Effect of substrate-
Table-1: Effect of NBA on the reaction rate.
|
NBA × 10-2M |
K × 10-3 |
|
1.0 |
4.559 |
|
1.5 |
4.553 |
|
2.0 |
4.549 |
|
2.5 |
4.546 |
|
3.0 |
4.540 |
|
3.5 |
4.528 |
A change in the initial concentration of malic acid resulted increase in the rate constant (Table 2) a plot of k1 versus [MA] at different temperatures are straight lines indicating that the reaction is first order with respect to [MA]
Effect of perchloric acid-
Table 2: Effect of Substrate, HClO4 , CH3COOH on the reaction rate.
NBA =0.02 M; [Hg(AcO2)] = 0.002M; Temp. 313K
The effect of [H + ] on the reaction rate was studied at fixed ionic strength maintained by sodium perchlorate. An increase in [HC1O4] lead to a decrease in the pseudo-first-order rate constants (kobs) and the plots of (1/ kobs) versus [HC1O4] were linear.
Effect of Variation of dielectric constant and anisamide-
The effect of dielectric constant of reaction medium was studied by adding acetic acid in the reaction medium at constant concentration of other reactants. Addition of anisamide (one of the reaction product) at constant NBA and malic acid conc. decreases the rate of reaction.
Activation parameters.
The reaction has been studied in the temperature range 313-328 K and the results are recorded in (table-3). Using Arrhenius equation the energy of activation for substrate has been calculated this value has been subsequently utilized in computing various other thermodynamic parameter and all the results are presented in (table-4).
Table-3: Effect of Temperature, on the reaction rate.
|
Temperature K |
K × 10-3 |
|
313 |
4.549 |
|
318 |
5.879 |
|
323 |
9.182 |
|
328 |
12.070 |
Table-4: Activation parameters for the oxidation of malic acid.
|
Substrate |
Ea (k.J1mol-1) |
ΔH* (k.J1mol-1) |
ΔS* (Jk-1mol-1) |
ΔG* (kJ.mol-1) |
|
Malic Acid |
59.06 |
56.45 |
101.51 |
88.23 |
Reaction Mechanism
Based on the above mentioned experimental observation, the following mechanism is suggested in which the protonated hydroxy acid react with active form of the oxidant HOBr in the rate determining step. The proposed mechanism for the reaction is
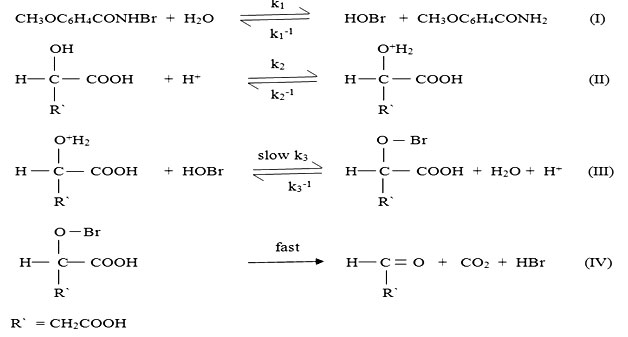
From the above mechanism, the following rate law is derived.
Rate = K1 K2 K3 [NBA][H+][Sub] [Anisamide]
kobs = K1 K2 K3 [H+] [Sub] [Anisamide]
which support the observed kinetic parameter like first order with respect to [substrate], [oxidant] and [H+] and inhibition by anisamide and also non-involvement of water molecule in rate determining step.
Acknowledgement
The authors are thankful to the Principal Dr. L. L. Dubey Govt. M.G.M. P.G. College Itarsi (M.P.) for providing laboratory facilities.
References
- Mohamed Farook, N. A. 2006. J. Iran. Chem. Soc. 3(4): 378-386
- Mohamed, Z. B. and Mohamed, F. A. 1953. J. Am. Chem. Soc. 75(22): 5731.
- Bishnoi, M. L., Negi, S.C. and Banerji, K. K.1988. Ind. J. Chem. 25A: 660.
- Saxena, R. and Upadhyay, S. K. 1991. Trans: Met. Chem. 16: 245-248.
- Madhu, S., Gupta, R., Singh, A., Singh, B. and Singh, A. K. 1991J. Mol. Chem. 65: 317.
- Singh, D. and Prasad, S. 2009. J. Chemtracks 11(1): 113-118.
- Patil, S., Katre, Y. R. and Singh, A. K. 2007 J. Surfa.and det. 10.1007/S 11743-007-1028-4: 1-21.
- Vijaya Mohan, K., Rao, P. R. and Sundram, E. V. 1988. Proc. Nat. Acad. Sci. India. 58(A): 4956.
- Singh, S. K., Gupta, H. D., Khan, M. U. and Baghel, S. S. 2010. Orbital Elec. J. Chem. Campo Grande, 2(2): 118-126
- Somanalli, K. R., Sannaiah, A., Kikkeri, N. and Rangaswamy, M. 2004. Czech. Chem. Commun. 69(8): 1577.
- Mathur, A., Sharma, V. and Banerji, K.K. 1988. Ind. J. Chem. 27A: 123.
- Jain, A. L. & Banerji, K. K (1982) J. Indian Chem. Soc. 59: 654.
- Banerji, K.K. 1977. Ind. J. Chem. 15A: 615.

This work is licensed under a Creative Commons Attribution 4.0 International License.









