Glutamic acid as an Efficient Catalyst for Synthesis of Dihydropyrimidinones
Ehsan Abbasi*and Farhad Hatamjafari
Department of Chemistry, Faculty of Science, Islamic Azad University-Tonekabon Branch, Tonekabon, Iran.
Corresponding author E-mail: abbasiehsan21@yahoo.com
A simple, efficient protocol for the one-pot Biginelli condensation reaction of aromaticaldehydes, β-ketoesters and urea employing glutamic acid as a novel catalyst is described.
KEYWORDS:Glutamic acid; Biginelli reaction; Dihydropyrimidinones; One-pot
Download this article as:| Copy the following to cite this article: Abbasi E, Hatamjafari F. Glutamic acid as an Efficient Catalyst for Synthesis of Dihydropyrimidinones. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Abbasi E, Hatamjafari F. Glutamic acid as an Efficient Catalyst for Synthesis of Dihydropyrimidinones. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22232 |
Introduction
Over the last decade, the multi-stage reactions (MCRs) became more popular. Multi-step synthesis to produce massive amounts of waste, according to the isolation of the complex, toxic and hazardous solvent at each step. So, MCRs will discovered, economically and environmentally friendly. Due to numerous advantages, these heavy compound molecules synthesized by the reaction of small molecule. Biginelli reaction is one of MCR that considerable attention recently1.
Dihydropyrimidinones (DHPMs) and their derivatives, as well as heterocyclic units in the field of natural and synthetic organic chemistry due to their wide range of biological and therapeutic properties such as anti-inflammatory, anti-tumor, anti-viral and anti-bacterial activities2-3. Recently, DHPM appropriate analogue functionalized gland known as the integral backbone of several calcium channel blockers, antihypertensive agents, and receptor antagonists have emerged. In addition, several alkaloids containing the dihydropyrimidine core unit have been isolated from marine sources also show interesting biological properties4. The most straightforward method for the synthesis DHPMs first reported by Biginelli more than 100 years ago, it consisted of three one-pot condensation of benzaldehyde, ethyl acetoacetat and urea under strongly acidic conditions5. However, these reactions often require harsh conditions and long reaction time and low efficiency can. Aliphatic and aromatic aldehydes, especially when used to replace for synthesis Dihydropyrimidinones (DHPMs).
Although numerous methods are capable of affecting these synthesis has been previously reported6-15. Glutamic acid has been used previously as a catalyst for synthesis of organic compound16, Previously, we have synthesized a number of heterocyclic compounds17-26. Herein we report glutamic acida new catalyst for the synthesis of DHPMs at one pot reaction, environmentally friendly with high yields and easy separation (Scheme 1).
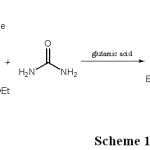 |
Scheme 1
|
Experimental
General Procedure for the Preparation of ethyl 1,2,3,4-tetrahydro-6-methyl-2-oxo-4-phenylpyrimidine-5-carboxylate
A mixture of ethyl acetoacetate (1 mol), benzaldehyde (1 mol) and urea (1 mol) and glutamic acid (0.05 g) with 10 ml acetonitrile was refluxed for 1 h. All reactions were monitored by TLC and the obtained solid was filtered, the solid was washed with water and recrystallized using absolute ethanol.
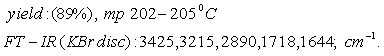
Results and Discussion
Herein, we report glutamic acid as catalyst which could provide an efficient, environmentally friendly, easy separation, high yield and simple route for the synthesis of DHPMs.
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of Tonekabon Branch Islamic Azad University.
References:
- Balme G., Bossharth E and Monteiro N., Eur. J. Org.Chem., 21: 4101 (2003).
- Kappe, C.O. Tetrahedron 1993, 49, 6937 ().
- Kappe, C.O. Acc. Chem. Res. 2000, 33, 879 ().
- Snider, B.B.; Chen, J.; Patil, A.D.; Freyer, A. Tetrahedron Lett. 1996, 37, 6977 ().
- Biginelli, P. Gazz. Chim. Ital. 1893, 23, 360 ().
- Banik, B.K.; Reddy, A.T.; Datta A.; Mukhopadhyay C. Tetrahedron Lett. 2007, 48, 7392 ().
- Li, J.T.; Han, J.F.; Yang, J.H.; Li, T.S. Ultrason. Sonochem. 2003, 10, 119 ().
- Peng, J.J.; Deng, Y.Q. Tetrahedron Lett. 2001, 42, 5917 ().
- Ma, Y.; Qian, C.; Wang, L.; Yang, M. J. Org. Chem. 2000, 65, 3864 ().
- Ahmed, N.; Lier, J.E.V. Tetrahedron Lett. 2007, 48, 5407 ().
- Adib, M.; Ghanbary, K.; Mostofi, M.; Ganjali, M.R. Molecules 2006, 11, 649 ().
- Karade, H.N.; Sathe, M.; Kaushik, M.P. Molecules 2007, 12, 1341 ().
- Cheng, J.; Qi, D.Y. Chin. Chem. Lett. 2007, 18, 647 ().
- Liu, C.J.; Wang, J.D.; Li, Y.P. J. Mol. Catal. A Chem. 2006, 258, 367 ().
- Zhang, X.L.; Li, Y.P.; Liu, C.J.; Wang, J.D. J. Mol. Catal. A Chem. 2006, 253, 207 ().
- Tanaka M., Ishimori K and Morishima I., Biochemical and Biophysical Research Communications, 227: 393 (1996).
- Bidram A., Hatamjafari F and Doryeh A., Orient. J. Chem., 29: (2013 in press).
- Azizian J., Hatamjafari F., Karimi A. R. and Shaabanzadeh M., Synthesis. 5: 765 (2006).
- Azizian J., Shaabanzadeh M., Hatamjafari F. and Mohammadizadeh M.R., Arkivoc., (xi): 47 (2006).
- Hatamjafari F., Synthetic Communications., 36: 3563 (2006).
- Azizian J., Hatamjafari F. and Karimi A. R., Journal of Heterocyclic Chemistry., 43: 1349 (2006).
- Hatamjafari F and Montazeri N., Turkish Journal of Chemistry., 33: 797 (2009).
- Hatamjafari F., Orient. J. Chem., 28: 141 (2012).
- Hatamjafari F., Orient. J. Chem., 29: (2013 in press).
- Hatamjafari F and Alijanichakoli F., Orient. J. Chem., 29: (2013 in press).
- Hatamjafari F and Hosseinian A., Orient. J. Chem., 29: (2013 in press).

This work is licensed under a Creative Commons Attribution 4.0 International License.









