Comparative Study of Kinetics of Catalyzed Oxidation of D (+)galactose and lactose by Ruthenium (III) in Alkaline Medium
Ashish Kumar
Chemical Laboratories, Lovely Professional University, India.
Article Received on :
Article Accepted on :
Article Published : 10 Jun 2013
Kinetic investigation in Ru(III) catalyzed oxidation of D(+)-galactose and lactose in an alkaline solution of potassium bromate in the presence of mercuric acetate as a scavenger for Br – ion has been carried out in the temperature range 30-45°C. The rate shows first order dependence with respect to the bromate and zeroth order with respect to the substrate (sugars). The reaction exhibits first order dependence on the catalyst ruthenium(III) and there is inverse order on the rate of reaction. Potassium chloride and acetic acid have a positive effect on the rate. Negligible effect of change in Hg(OAc)2, ionic strength of the medium and D2 O. [RuCl3 (H2 O)OH]-1 and BrO3 - are the most reactive species Ru(III) chloride and bromated, respectively. Galactonic acid and lactobionic acid have been identified as the main oxidation products of the reaction. Various activation parameters have bee calculated and recorded. On the basis of experimental findings, a suitable mechanism consistent with the observed kinetics was proposed and the rate law has been derived on the basis of obtained data.
KEYWORDS:Alkaline medium; Kinetics; Oxidation; Potassium bromate; Ru(III) catalyzed; Sugars
Download this article as:| Copy the following to cite this article: Kumar A. Comparative Study of Kinetics of Catalyzed Oxidation of D (+)galactose and lactose by Ruthenium (III) in Alkaline Medium. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Kumar A. Comparative Study of Kinetics of Catalyzed Oxidation of D (+)galactose and lactose by Ruthenium (III) in Alkaline Medium. Available from: http://www.orientjchem.org/?p=11965 |
Introduction
Catalysis by transition metal ions, plays an important role in understanding the mechanistic aspects of a particular redox reaction. Ru(III) acts as an efficient catalyst in many redox reactions, hence the use of ruthenium(III) chloride as homogeneous catalyst in acidic medium.A number of oxidants like potassium bromate[1-2], N-bromoacetamide [3-5], N-bromosuccinimide[6,7], Chloramine-T [8] and sodium periodate [9-10] have been earlier used in oxidation of various compounds.The kinetics of redox reactions incorporating certain transition metals ions like Osmium(VIII) [11], ruthenium(VIII) [12], iridium(III) [13] and palladous ion as homogeneous catalyst has been extremely investigated. However, reactions involving Ru(III) [14-15] have been little investigated and hardly any investigation has so far been reported to explain the catalytic role of ruthenium(III) chloride with potassium bromate as an oxidant in alkaline medium. This propmted us to undertake the present investigation which consists of ruthenium(III) catalyzed oxidation of D(+)-galactose and lactose in nano concenttration range by alkaline potassium bromate in the presence of mercuric acetate as a scavenger.
Objectives of the present study are to: elucidate a plausible mechanism, identify the oxidation products, deduce an appropiate rate law, ascertain the various reactive species and calculate activation parameters.
Experimental
Material
An aqueous solution of D(+)-galactose and lactose (E.Merck), potassium bromate (BDH; AR), NaOH and NaClO4 (E.Merck) were prepared by dissolving the weighed samples in triply distilled water. NaOH was used as a source of OH– ion. Sodium perchlorate was used to maintain the ionic strength of the medium. A solution of RuCl3 (Johnson Matthey) was prepared in HCl of known strength. Deuterium oxide (D2O) (purity 99.4%) was supplied by BARC Mumbai, India. All other reagents were of analytical grade and stills employed were of Jenna glass. Reaction vessels were painted black so as to prevent photochemical decomposition, if any.
Kinetics
A thermostatic water bath was used to maintain the desired temperature within ±0.10C. Requisite volume of reagents, including substrate, was taken in a reaction vessel and thermostated at 35 ± 0.10C for thermal equilibrium. A measured volume of oxidant solution, which was also maintained separately at the same temperature, was rapidly poured into the reaction vessel. The kinetics was followed by examining desired portions of reaction mixture for oxidant iodometrically using starch as an indicator after suitable time intervals.
Stoichiometry
The stoichiometry of the reaction was determined by equilibrating varying ratios of [bromate] to sugars at 350 C for 48 hrs under kinetic condition. Estimation of unconsumed bromate revealed that, one mole of the substrate consumes one mole of the oxidant. The oxidation of D(+)-galactose and lactose leads to the formation of galactonic acid and lactobionic acid respectively. The stoichiometric determination indicated the following overall reaction:
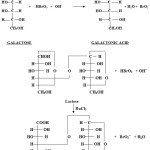 |
Scheme 1 Click here to View scheme |
Identification of end product formed in the above reaction i.e. corresponding acid16 was carried out as follows-
Neutralized 5 ml of acid with excess of ammonia in a boiling test tube. Then boiled the solution to remove excess of ammonia, cooled and added few drops of neutral of FeCl3 solution. A reddish brown colour ppt. is obtained, which confirms presence of carboxylic group.
Results and Discussion
Ruthenium(III) catalyzed oxidation of D(+)-galactose and lactose was investigated at several initial reactant concentrations (Table-1). First order kinetics was observed in case of oxidant, i.e. bromate which was also confirmed by a plot of (-dc/dt) versus [Bromate] (Fig.1). The plot obtained is linear with a slope value of 3.33 for D(+)-galactose and 3.36 for lactose, which is similar to the average k1 3.46 & 3.48 respectively for D(+)-galactose and lactose values. Zero order dependence on substrate was established by varying the amount of D(+)-galactose and lactose at fixed concentration of all other component. Variations in hydroxide ion show negative effect, which means that the rate of the reaction decreases with increase in [OH–] concentration while variation of chloride ion concentration shows positive effect (Table-1). The reaction was markedly influenced by increase in ruthenium(III) concentration. A plot of log (-dc/dt) versus log [Ru(III)] (Fig. 2) gives a slope 1.16 at 350C for D(+)-galactose and 0.923 at 350C for lactose which further confirms first order dependence on Ru(III).
![Figure 1: Plot between [KBrO3] x 10-3 M vs. (-dc/dt) x 10-7 ML-1s-1 for oxidation of D(+)-galactose (A) and lactose (B) at 350C.](http://www.orientjchem.org/wp-content/uploads/2013/06/Vol29No2_Comp_Ashi_fig1-150x150.jpg) |
Figure 1: Plot between [KBrO3] x 10-3 M vs. (-dc/dt) x 10-7 ML-1s-1 for oxidation of D(+)-galactose (A) and lactose (B) at 350C.
|
![Figure 2 : Plot between 6 + log [Ru(III)] vs. 8 + log (-dc/dt) for oxidation of D(+)-galactose (A) and lactose (B) at 350C.](http://www.orientjchem.org/wp-content/uploads/2013/06/Vol29No2_Comp_Ashi_fig2-150x150.jpg) |
Figure 2 : Plot between 6 + log [Ru(III)] vs. 8 + log (-dc/dt) for oxidation of D(+)-galactose (A) and lactose (B) at 350C. |
Kinetics results (Table-1) show negligible effect of ionic strength of the medium (affected by addition of NaClO4) and addition of Hg(Oac)2. The rate measurements were taken at 300– 450 C and specific rate constant were used to draw a plot of log k vs I/T, which was linear (Fig.3). The values of energy of activation (ΔE*), Arrhenius factor (A), entropy of activation (ΔS*) and free energy of activation (ΔG*) were calculated and these values have been recorded in Table-2.
 |
Figure 3 : Plot between 7 + log (-dc/dt) vs. 1/T x 104 K-1 for oxidation of galactose (A) and lactose (B) at 350C.
|
The negligible effect of mercuric acetate excludes the possibility of its involvement either as catalyst or as an oxidant. Hence its function is to act as a scavenger17-19 for any bromide ion formed in the reaction.
Ru(III) chloride has been reported to give a number of possible chloro species dependent on pH of the solution, Ru(III) exists in the following equilibrium under the experimental pH range 10 – 1220. Our experimental data indicate that addition of chloride ion has a positive effect on the reaction velocity.
![]()
In alkaline solution of potassium bromate quick formation of BrO3– ion has been reported. The above statements lead us to suggest the following reaction scheme, which gives the details of various steps in the reaction.
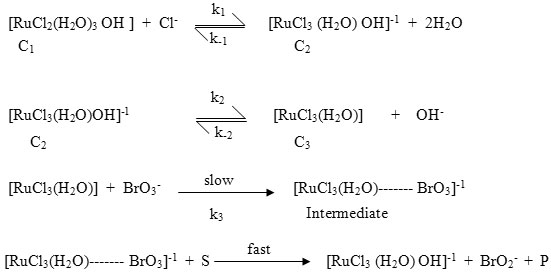
where S = substrate P = corresponding acid products
Considering the above slow steps and applying steady state treatment with a reasonable approximation, the rate law for D(+)-galactose and lactose may be written in terms of consumption of [BrO3–] as equation:-

where K1 = (k1/k-1) and K2 = (k2/k-2)
The rate law is in agreement with all observed kinetics. The proposed mechanism is in consistent with the activation parameters given in table-2. The high positive values of change in free energy of activation (∆G*) indicates highly solvated transition state, while fairly high negative values of change in entropy of activation (∆S*) suggest the formation of an activated complex with reduction in the degree of freedom of molecules.
Table 1.1: Effect of variation of substrate and oxidant on the reaction rates for sugars at 350C
|
[Substrate ] x 102 M
|
[KBrO3] x 103 M
|
[KCl] x 103 M |
[NaOH] x 103 M |
[Hg(OAc)2] x 103 M |
(-dc/dt) x 107 ML-1 s-1
|
|
| D(+)-galac. | Lactose | |||||
|
0.33 0.40 0.50 0.66 1.00 2.00 |
1.00 1.00 1.00 1.00 1.00 1.00 |
1.00 1.00 1.00 1.00 1.00 1.00 |
1.00 1.00 1.00 1.00 1.00 1.00
|
1.25 1.25 1.25 1.25 1.25 1.25
|
3.57 3.30 3.40 3.75 3.69 3.48 |
3.80 3.75 3.71 3.60 3.60 3.60 |
|
1.00 1.00 1.00 1.00 1.00 1.00
|
0.83 1.00 1.25 1.66 2.50 3.33
|
1.00 1.00 1.00 1.00 1.00 1.00 |
1.00 1.00 1.00 1.00 1.00 1.00
|
1.25 1.25 1.25 1.25 1.25 1.25
|
2.77 3.69 4.40 6.00 8.00 11.42 |
2.75 3.60 4.50 5.50 8.57 12.00 |
[Ru(III)] = 0.096 x 10-9 M (D(+)-galactose) & 0.0288 x 10-9 M(lactose).
Table 1.2: Effect of variation of reactants on the reaction rates for sugars at 350 C
|
[KCl] x 103 M |
[NaOH] x 103 M |
[Hg(OAc)2] x 103 M |
[NaClO4] x 103 M |
(-dc/dt) x 107 ML-1 s-1D(+)-galac. Lactose |
|
1.00 1.00 1.00 1.00 1.00 1.00 0.83 1.00 1.25 1.67 2.50 5.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 |
1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 0.83 1.00 1.25 1.67 2.50 5.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 |
1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 0.83 1.00 1.25 1.67 2.50 5.00 1.25 1.25 1.25 1.25 1.25 1.25 |
– – – – – – – – – – – – – – – – – – – – – – – – 0.83 1.00 1.25 1.67 2.50 5.00 |
3.57 3.803.30 3.753.40 3.71
3.75 3.60 3.69 3.60 3.48 3.60 2.84 3.45 3.69 3.60 3.85 3.71 4.00 4.33 4.50 4.66 4.63 5.00 4.15 4.20 3.69 3.60 3.40 3.50 2.85 3.40 2.76 3.27 2.60 2.92 3.50 3.23 3.25 3.83 3.69 3.60 3.70 4.00 3.13 3.45 3.69 3.60 3.72 3.70 3.50 3.63 3.47 3.75 3.66 3.55 3.80 3.60 3.75 3.68 |
[KBrO3] = 1.00 x 10-3 M,
[Substrate] = 1.00 x 10-3 M
[Ru(III)] = 0.096 x 10-9 M (for D(+)-galactose) & 0.0288 x 10-9 ( for lactose).
Table 2: Activation parameters for alkaline bromate oxidation of D(+)-galactose and Lactose.
|
Substrate |
Temperature coefficient |
ΔEa kJ mol-1 |
ΔH* kJ mol-1 |
ΔS* JK mol-1 |
ΔG* kJ mol-1 |
Log A |
kr mol-2 lit-2 sec-1 |
|
D(+) galactose lactose |
2.03 1.99 |
57.66 54.66 |
72.70 69.92 |
-15.26 -15.10 |
77.40 74.57 |
9.66 9.70 |
0.84 2.92 |
Conclusions
The experimental results as shown reveals that the reaction rate doubles when the concentration of the catalyst [Ru(III)] is doubled. The rate law is in conformity with all kinetic observations and the proposed mechanistic steps are supported by the negligible effect of ionic strength. From this investigation, it is concluded that BrO3– and [RuCl3(H2O)OH]-1 are the reactive species of KBrO3 and Ru(III) chloride respectively in alkaline medium.
References
- Sheila Srivastava, Parul Srivastava , Ashish Kumar and Shalini Singh ; Bulletin of the Catalysis Society of India , 6, 119-124 (2007).
- Mridula Sharma, Gayatri Sharma , Beena Agrawal, C.L. Khandelwal and P.D. Sharma; Transition Metal Chemistry., 30(5), (2005), 546-551.
- Sheila Srivastava, Vartika Srivastava, Ajaya Awasthi; Oxid. Commun., 26 No-3, (2003), 378-384.
- Ashok K.Singh, Amar Singh, Ranjana Gupta, Madhu Saxena, Bharat Singh; Transition Met. Chem. (Landon)., 17(5), (1992), 413-16 (Eng).
- Sheila Srivastava, Ajaya Awasthi, Vartika Srivastava; Oxid. Commun., 26 No-3, (2003), 426-431.
- K.Surekha Mavalang, Miss Nirmal, N-Halligudi, T-Sharanappa Nandbewoor; Reaction Kinetic and Catalysis Letters., 72(2), (2001), 391-398.
- A.K.Singh, Deepti Chopra; Oxid.Commun., 20(3), (1997), 450-464.
- Sheila Srivastava and Pushpanjali ; Bulletin of the Catalysis Society of India , 7 , 12-19 (2008)
- Neena Gupta, Shahla Rahmani, A.K.Singh; Oxidation Communication., 22(2), (1999), 237-243 .
- R.D.Kaushik, Shashi, Amrita, Sumetra Devi; Asian J. Chem., 16(2), (2004), 818-22.
- Veeresh Seregar, T.M. Veeresh and Sharanappa T. Nandibewoor ; Journal of Molecular Catalysis: A Chemical., 271(1-2), (2007), 253-260.
- Sheila Srivastava, Sarika Singh and Parul Srivastava ; Asian J. Chem., 20 (1), 317-323 (2008).
- B.Singh, M.Singh, P.Bhatnagar, A.Kumar ; Oxid. Commun., 17,48 (1994).
- Sheila Srivastava, Pooja Khare , Shalini Singh and Parul Srivastava ; Bulletin of the Catalysi Society of India, 7, 1-11 (2008).
- Sheila Srivastava , Kulina Singh , Ajaya Awasthi , Shalini Singh and Parul Srivastava ; International J. of Pure & Applied Chemistry , 2(4), 391-395 (2007).
- Arun Sethi ; Lab Experiments In Organic Chemistry (2003).
- Sheila Srivastava , Bharat Singh ; Trans. Metal Chem., 16 ,466 (1991)
- N. Venkata Subramanian , V. Thigarajan ; Canadian J. Chem., 47 , 694 (1969).
- J. C. Bailer , “ Chemistry of Coordination Compound ; Reinhold , New York (1964), P-4.
- A.K.Singh, N.Chaurasia, S.Rahmania, J.Srivastava and B.Singh; Catalysis Letters, 95,135-142 (2004).

This work is licensed under a Creative Commons Attribution 4.0 International License.









