Comparative of Isotherms Adsorption Methyl Orange and Phenolphthalein by Multi-wall Carbon Nanotube and Activated Carbon with Models Freundlich, Temkin and Langmuir
Mehdi Vadi* and Vahid Namavar
Department of Chemistry, Firozabad Branch, Islamic Azad University, Firozabad, Fars, Iran. Correspondence author E-mail: mahdi_vadi@iaufasa.ac.ir
Adsorption of methyl orange and phenolphthalein by multi-wall Carbon Nanotube and activated carbon is very important, because methyl orange is used widely in acid-basic indicators and phenolphthalein as an indicator sodium or potassium alkoxide or titration of carboxylic acids hydroxide. Adsorbed compounds depend on sample concentration. Isotherms adsorption methyl orange and phenolphthalein with very models are studied. The purpose of this study was to investigate adsorption isotherms of methyl orange and phenolphthalein by multi-wall Carbon Nanotube (MWCNTs) and activated carbon (AC) with models Freundlich ,Temkin , Langmuir . By maximum wavelength is obtained by spectrophotometer (uv / vis) model (JENWEY) and different concentrations are obtained from made solution and their adsorptions, and relative diagram was drawn. Study of experiments result was matched with these three models and different parameters of these models were obtained. Obtained results show the effect of concentration on covering the surface by model Langmuir for active carbon by methyl orange with 95.3% and model Freundlich for active carbon by phenolphthalein with 98.7% and model Freundlich for Carbon Nanotube by methyl orange with 96.3% and model Langmuir for Carbon Nanotube by phenolphthalein with 95.5% . Results of this study show that Priority of model of models for adsorption of methyl orange by Carbon Nanotube is : 1) Freundlich , 2) Temkin , 3) Langmuir and for adsorption phenolphthalein by Carbon Nanotube is : 1) Langmuir , 2) Freundlich , 3) Temkin and for adsorption of methyl orange by active carbon : 1) Langmuir , 2) Temkin , 3) Freundlich and for adsorption of phenolphthalein by active carbon : 1) Freundlich , 2) Temkin , 3) Langmuir .
KEYWORDS:Isotherm; adsorption; methyl orange; phenolphthalein; Multi-wall Carbon Nanotube; active carbon
Download this article as:| Copy the following to cite this article: Vadi M, Namavar V. Comparative of Isotherms Adsorption Methyl Orange and Phenolphthalein by Multi-wall Carbon Nanotube and Activated Carbon with Models Freundlich, Temkin and Langmuir. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Vadi M, Namavar V. Comparative of Isotherms Adsorption Methyl Orange and Phenolphthalein by Multi-wall Carbon Nanotube and Activated Carbon with Models Freundlich, Temkin and Langmuir. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22047 |
Introduction
Carbon nanotubes(CNT) are different form of carbon with a cylindrical nano-structure . Nanotube with the length-to-diameter ratio to 132,000,000:1 is made very larger than any other materials. These cylindrical carbon molecules have unusual characteristics which are very important for nano technology, electronic, optic and other aspects of material and technology of materials. Specifically, can be found the thermal and mechanical properties and electrical conductivity of carbon nanotubes on the extraordinary applications as additives to various structural materials. Multi-wall Carbon nanotubes can absorb many atoms and molecules on their surfaces such as metallic elements like lithium , potassium , rubidium , cesium and non-metallic elements such as hydrogen ,oxygen , nitrogen and methanol . Characteristic of nanotube is adsorption of gases such as hydrogen and other gases. All of these compound on the surface of adsorption nanotube are two main parts of Covalent and non-Covalent bonds [1] . Carbon nanotubes are very attractive, because they can emit upper flows to (1 A/cm2) in down domain (5 V/µm) and show their abilities as transmitter in different devices which are show in five past years. In addition, controlled deposition of nanotubes on the substrate may be synthetic by using of chemical vapor deposition (CVD) methods andhavebecomecatalysts [2].
Active carbon is used widely as adsorbent , catalyst and catalyst in various industrial applications and environmental ( for example processes treatment, recovery chemical products and removal of organic and metals) the ability to adsorption and catalytic activity greatly their surface properties controlled has been shown for example carbon used for adsorbent of gases and vapor , should be The effective radius with pore degree , significantly smaller than 16-20 Å while active carbon for with transitional porosity range is 20-500 Å as absorbents significantly to omit the color non-pures from the liquid phase system [3] . Active carbon which is derived from the porous polymers , forms new kind of carbon adsorbent gives them advantageous Characteristics of such as well-developed micro- and mesoporous structure , high adsorption capacity , high mechanical strength and spherical in shape of granules [4] .
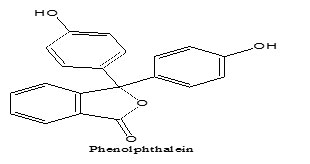
Phenolphthalein is a yellowish-with crystal powder, odorless and unstable in air. Insoluble in water and it solves in alkaline solution. Alcohol solutions are used as an indicator. pH Phenolphthalein transmission distance is between pH 8.2 (colorless) and pH 9.8 (violet) . Phenolphthalein can be used indicator as the sodium or potassium alkoxide or titration of hydroxide carboxylic acids and other acidity compounds in benzene, toluene, pyridine, ketones and other solvents. In the water it similar color change but often Phenolphthalein changes its color in some non-aqueous solvents. It is known as Di-p-dioxydiphenylphthalide . Its molar mass 318.3 and it formula C20H14O4 and shape structural is bellow:
Methyl orange is a yellow- orange powder in crystal scales . The substance is soluble in water and actually insoluble in alcohol . Aqueous solvent 0.04% which is used as an indicators solution . It pH transition range methyl orange is between (pH 3.1 red) and (pH 4.4 yellow) . Methyl orange is widely used in acid-basic indicators and usually show the end point of titration strong acids , strong and weak base is used and the names of 4′-Dimethylaminoazobenzene-4-sulphonic acid (Na-salt), helianthin, helianthin B, tropeolin D, orange III, gold orange is know .Its molar mass 327.3 and formula C14H14N3O3SNa and shape structural is bellow :
Error methyl orange is that in solution with higher temperature transition range pH change is significant . Determine the pH color of methyl orange initially recommended by Sorensen and is still in used [5] .
Experimental
Us in this first tested the methyl orange distilled by water and Phenolphthalein distilled by water and alcohol solution with ratio 60 to 40 and with a concentration 100ppm in 250ml Volumetric flask standards have made . Then concentrations of 10, 20, 30, 40, 50 and 60ppm in 50ml Volumetric flask by the equation M1V1 = M2V2 standard’ve made. Then10 mlofeach oftheconcentrationsthe 3test tubeAs suchthatthetubefirstshed10 mlofPhenolphthalein solutionmade of without carbon nanotubes and activated carbon, In the amount of second experiment tubes 0.002 g of carbon nanotubes shed into a test tube and then add 10 ml of the above solution smooth it by filter paper and the amount of third test tube 0.002 g of activated carbon shed into test tube and then add 10 ml of the above solution smooth it by filter paper. Absorption related to Phenolphthalein concentrations of 100ppm taken and the max Landa notes, and then according to the max Landa and also reset to zero the device Witness by solution, we have measured the Absorption of each concentration for orange methyl we act the same way. It should be noted that the Witness solution of for Phenolphthalein without carbon nanotubes and activated carbon respectively solution of distilled water and alcohol and for methyl orange is distilled water. When add activated carbon or carbon nanotubes solution Witness distilled water and alcohol containing and activated carbon or carbon nanotubes is by filter paper are smooth.
Below 3 Image of concentrations 20 ppm Phenolphthalein absorption by activated carbon and carbon nanotubes have been shown.
 |
Figure 1
|
 |
Figure 2
|
 |
Figure 3 |
Materials and Methods
Devices and Tools
In this research spectroscopic device for determining the concentration of uv / vis, analytical balance (Mettler H 30) with an accuracy of 0.0001 gram air Volumetric flask 250 and 50 ml , experiment tube and funnel filter paper is used.
Chemical material
Multi-walled carbon nanotubes with high purity 95% and activated carbon and Phenolphthalein and methyl orange were purchased from Aldrich. Merck has been used to prepare the company.
Results
The equilibrium adsorption isotherms:
Equilibrium relationships between adsorbent and adsorption are defined by isotherms adsorption that show the relationship between the amount of adsorbed material and some of it remains in the balance.
Langmuir model
This adsorption model is used to adsorption a single layer. All these models assume the same energy adsorption sites on the surface of the adsorbent, the linear form is expressed by the equation [6] .
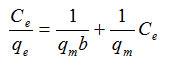
Where qe is the amount of adsorbed from solution (mgg-1) and Ce concentration in solution at equilibrium (mgL-1) and Langmuir constants qm and b are constants that represent the equilibrium adsorption capacity and adsorption layer is saturated.
Freundlich model
Freundlich isotherm is an empirical equation to further understanding of metal ion adsorption on heterogeneous surface by multilayer adsorption and adsorption of the solved amount of the infinite increase the concentration of most [7] Freundlich adsorption isotherms are described by the following equation:
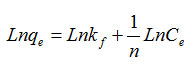
In this equation, qe is the adsorption solved (mgg-1) and Ce concentration in solution at equilibrium (mgL-1) and the constants n and kf is respectively indicating the adsorption intensity and adsorption capacity.
Temkin model
Temkin isotherms contain a factor that interaction between the adsorbent and particles adsorb to clearly indicate [8-9]. Temkin in to these isotherms are linear forms:
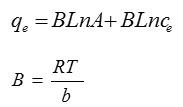
In this relationship A terms of (L / mg) is equal to a constant link associated with the maximum binding energy, b in terms of the (J / mol) isotherms temkin constant and B (without unit) is proportional to the heat of adsorption.
Process adsorption isotherm of phenolphthalein and methyl orange on the carbon nanotubes and activated carbon are shown in Figures 1 to 12 and calculated the parameters of these models are shown in Table 1.
Table 1: Parametersandcorrelation coefficients ofisothermmodels
|
Temkin model |
Freundlich model | Langmuir model | |||||||
| A(L/mg) | R2 | B | R2
|
n
|
K(mg/g)
|
b(L/mg) | R2 | Q(mg/g)
|
|
| 0.66 | 0.925 |
34.19 |
0.874 | 1.8 | 15.9 | 0.0814 | 0.953 | 142.86 | Activated carbon by methyl orange |
| 0.24 | 0.928 |
5.318 |
0.963 | 1.63 | 1.22 | 0.022 | 0.868 | 25.64 | Carbon nanotubes by methyl orange |
| 1.05554 | 0.957 |
79.46 |
0.987 |
1.4 |
35.77 | 0.069 | 0.948 | 500 | Activated carbon by phenolphthalein |
| 9.96 | 0.76 |
5.539 |
0.805 |
5.377 |
16.56 | 0.125 | 0.955 | 41.67 | Carbon nanotubes by phenolphthalein |
Conclusions
Considering that adsorption onto a solid surface due to attractive forces between functional groups on the solid surface and molecules material is absorbed , We reached to the conclusion that attracts methyl orange on the carbon nanotubes is better than adsorption of phenolphthalein because it methyl orange structure is small and has less space to prevent (less benzene ring) and methyl orange functional groups linked to better together and with form a multi layer on the active sites, has been absorbed. Carbon nanotubes are multi-layer structure and due to their pores and also small methyl orange , amount of methyl orange each layer of adsorbed but , phenolphthalein due to large structure and space to prevent failed to establish a strong link and the smaller amount of on to layers of carbon nanotubes has been absorbed .
Methyl orange adsorption onto activated carbon is better than phenolphthalein absorbed . Activated carbon does not definite arrangement and because it methyl orange structure is small and has less space to prevent and methyl orange functional groups are linked to better together and with on the place to active as single layer of adsorbed but , phenolphthalein due to large structure and space to prevent the smaller amount of on to layers of carbon nanotubes has been absorbed .
Methyl orange adsorption onto CNT and activated carbon (AC) into the attractive functional groups SO3 and pair of electrons non-link oxygen , which increases the resonance and negative charge density and acidic nature increases the Its absorption intensity increases but phenolphthalein due to the strong electron groups (OH) more , acidic nature decreases and lowers the absorption intensity. Methyl orange has the Aromatic compounds is less than phenolphthalein. According to the results obtained in the case of CNT and activated carbon (AC) investigated in this research, Langmuir models for adsorption methyl orange by activated carbon and freundlich model for absorption phenolphthalein by carbon nanotubes is consistent . Langmuir model represents is adsorption for attract single layer and same energy all sites absorb the on the adsorbent surface and freundlich model represents Ions adsorption on heterogeneous surfaces with multi-layer adsorption and adsorbed amount increases with increasing concentration.
References
- M.Vadi , Adsorption Isotherms of Some Non-Steroidal Drugs on Single Wall Carbon Nanotube , Asian Journal of Chemistry; Vol. 25, No. 6 (2013), 3431-3433
- J-M. Bonard , M. Croci , Ch. Klinke , R. Kurt , O. Noury , N .Weiss , Carbon nanotube films as electron field emitters , Carbon 40 (2002) 1715–1728
- N R .Khalili , M . Campbell , G . Sandi , J . Golas , Production of micro- and mesoporous activated carbon from paper mill sludge I. Effect of zinc chloride activation , Carbon 38 (2000) 1905–1915
- A.M. Puziy , O.I. Poddubnaya , A. Martinez-Alonso , F. Suarez-Garcia , J.M.D. Tascon ,Synthetic carbons activated with phosphoric acid II. Porous structure , Carbon 40 (2002) 1507–1519
- edmund bishop , 1 ed, Book indicators , 1972 , pergamon press, germany
- F.Martinez,A.Hernandez,”Protein adsorption onto microfiltration membranes:The role of solute-solid interactions”,J.Colloid and Intreface Sci.,221،254-2612،2006
- Jeong-yeolyoon,woo-sikkim, ”Adsoption of BSA on highly carboxylate microspheres quantitative effects of surface functional groups and interaction forces‖,J.Colloid and Interface Sci.,177 ،613-620 ،1996
- M. Özacar, İ.A. Şengil, Bioresour. Tecnol., 96 (2005) 791-795.
- I.D. mall, V.C. Srivastava, N.K. Agarwall, I.M. Mishra. ChemospHere, 61(2005) 492-501.

This work is licensed under a Creative Commons Attribution 4.0 International License.










