Antimicrobial and Pharmacological Studies of some Newly Synthesized Aryl Sulfonamide Derivatives
Hanan A. Abdel Fattahattah attah
Department of Pharmacutical Organic Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt.
A series of some newly synthesized aryl sulfonamide derivatives containing pyrazole, oxadiazole, pyrrole and hexahydroquinoline moieties was prepared starting from 4-tosyl aminobenzohydrazide 4. The structure of the newly synthesized compounds were characterized by spectroscopic methods and elemental analysis. Some of the newly synthesized compounds were evaluated in vitro for antimicrobial, analgesic and anti-inflammatory activities. The antimicrobial activity was screened against Gram negative, Gram positive strains and fungi. The result of the antimicrobial activity revealed that most of the tested compounds exhibit antimicrobial and antifungal activities. In addition compound 12 g exhibits antifungal activity against both Aspergillus niger and Candida albicans greater than Nystatin. The analgesic and anti-inflammatory screening showed that compound 11 has higher activity than Celecoxib and Voltarin.
KEYWORDS:Antimicrobial; Synthesis; Aryl; Sulfonamides
Download this article as:| Copy the following to cite this article: Attah H. A. A. F. Antimicrobial and Pharmacological Studies of some Newly Synthesized Aryl Sulfonamide Derivatives. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Attah H. A. A. F. Antimicrobial and Pharmacological Studies of some Newly Synthesized Aryl Sulfonamide Derivatives. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22043 |
Introduction
Sulfonamide and their derivatives are used in medicine due to their pharmacological properties such as antibacterial activity(1,2). Oxadiazole derivatives belong to an important group of heterocyclic compounds that have a vital role in medicinal chemistry such as antibacterial(3,4), antifungal(4,5), anti- inflammatory(6) and analgesic(7,8). Moreover pyrrole and pyrazole derivatives bearing biologically active sulfonamide moiety were reported to exhibit antibacterial and antifungal activities(9). Also pyrazole derivatives have been reported as analgesic and ant-inflammatory(10). Quinoline and its derivatrives have attracted much more attention as an important class of heterocyclic compounds in the field of medicine and pharmaceutical as antibacterial and antifungal(11), analgesic(12) and anti-inflammatory(13,14). In this work novel aryl sulfonamide derivatives containing different heterocyclic moieties as pyrrole, pyrazole, 1,3,4-oxadiazole and quinoline derivatives were synthesized hopping to produce potent antibacterial, antifungal ,analgesic and anti-inflammatory with no or less tendency to evoke gastric ulceration.
Results and Discussion
Chemistry
The preparation of the new compounds is outlined in schemes 1&2. The starting compound 4-tosylaminobenzohydrazide 4 was prepared via reaction of ethyl4-tosylaminobenzoate with hydrazine hydrate 99% under reflux as reported method (15). The hydrazide 4 was reacted with 2,5-hexanedione in glacial acetic acid to give compound 5. Refluxing the hydrazide 4 with acetylacetone, benzoyl acetone and ethyl acetoacetate in ethanol containing drops of acetic acid gave compounds 6a, b and 7, respectively. Hydrazone 8 was prepared via refluxing the hydrazide 4 with 3,4,5-trimethoxybenzaldehyde in ethanol. Moreover stirring the hydrazide 4 with p-chlorobenzoyl chloride in glacial acetic acid yielded compound 9.
Compounds 10a-c were prepared by refluxing hydrazide 4 with maleic anhydride, dichloromaleic anhydride and phthalic anhydride in glacial acetic acid, respectively.
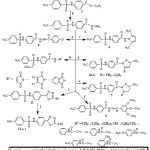 |
Scheme 1 Click here to View scheme |
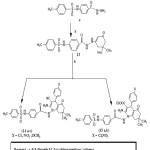 |
Scheme 2 Click here to View scheme |
Table 1: Antimicrobial activity of synthesized compounds by cup plate diffusion method
|
Samples |
Diameter (mm) of inhibition zones against the corresponding standard strains of different microorganisms |
||||||||
|
Gram-positive bacteria |
Gram-negative bacteria |
Fungi |
|||||||
|
Staphylococcus aureus ATCC6538 |
Staphylococcus epidermidiss ATCC12228 |
Micrococcus spp. ATCC10240 |
Pseudomonas aeruginosae ATCC9027 |
Klebsiella pneumoniae ATCC27736 |
Salmonella typhimurium ATCC14028 |
Escherichia coli ATCC10536 |
Aspragillus niger ATCC16404 |
Candida albicans ATCC10231 |
|
|
5 |
15 |
14 |
19 |
17 |
18 |
18 |
19 |
24 |
22 |
|
6a |
15 |
14 |
17 |
18 |
19 |
19 |
20 |
22 |
21 |
|
6b |
17 |
16 |
19 |
17 |
17 |
17 |
18 |
25 |
24 |
|
7 |
14 |
14 |
16 |
17 |
18 |
18 |
19 |
18 |
16 |
|
8 |
22 |
21 |
23 |
18 |
19 |
19 |
20 |
22 |
22 |
|
9 |
18 |
17 |
20 |
18 |
19 |
19 |
20 |
22 |
22 |
|
10a |
17 |
16 |
19 |
18 |
18 |
18 |
19 |
22 |
21 |
|
10b |
21 |
20 |
23 |
17 |
18 |
18 |
19 |
24 |
23 |
|
11 |
20 |
19 |
22 |
18 |
19 |
19 |
20 |
23 |
22 |
|
12a |
18 |
17 |
20 |
17 |
18 |
18 |
19 |
23 |
22 |
|
12b |
15 |
15 |
17 |
18 |
19 |
19 |
20 |
19 |
17 |
|
12c |
20 |
19 |
22 |
17 |
17 |
17 |
17 |
21 |
19 |
|
12d |
19 |
18 |
20 |
17 |
18 |
18 |
19 |
22 |
21 |
|
12f |
17 |
16 |
19 |
18 |
18 |
18 |
19 |
22 |
21 |
|
12g |
17 |
16 |
19 |
18 |
18 |
18 |
20 |
26 |
25 |
|
12h |
18 |
18 |
20 |
18 |
19 |
19 |
20 |
24 |
23 |
|
13 |
19 |
19 |
20 |
20 |
21 |
21 |
22 |
23 |
22 |
|
14a |
15 |
14 |
17 |
19 |
19 |
19 |
20 |
20 |
18 |
|
14b |
16 |
15 |
17 |
18 |
18 |
18 |
20 |
21 |
19 |
|
15a |
15 |
15 |
17 |
18 |
19 |
19 |
20 |
18 |
16 |
| Cefotaxime (Refrence) |
30 |
28 |
35 |
30 |
32 |
33 |
35 |
– |
– |
| Sulphamethoxazole (Refrence) |
25 |
23 |
30 |
25 |
27 |
28 |
30 |
– |
– |
| Nuystatin (Refrence) |
– |
– |
– |
– |
– |
– |
– |
25 |
20 |
| DMF (control) |
– |
– |
– |
– |
– |
– |
– |
– |
– |
- · The method used is cup plate diffusion method · Each cup is filled with 100 micro liter from each sample
- · Conc. Of each sample is 50 mg/ml · Conc. of antimicrobial agents is 5 mg/ml
Table 2: Analgesic activity evaluation. of compounds 5, 6a,b, 7, 8, 10a, 11, 12b-f, 13, 14a,b and 15a.
|
Group |
Reaction time in seconds after |
|||||
| Mean±SEM |
10 min. |
20 min. |
30 min |
60 min. |
90 min. |
120 min. |
| Control |
13.4±0.2 |
13.8±0.3 |
15.4±0.4* |
15.2±0.3* |
15.8±0.3* |
12.8±2.9 |
| Celecoxibe |
28.8±1.2# |
30.4±1.4# |
32.6±1.6# |
33.4±1.5*# |
34±1.1*# |
33.8±0.8*# |
| Diclofenac (Na.) Voltarin |
31.2±1.4# |
32±1.3# |
33.2±1.4# |
34.2±1.3# |
34.2±0.8# |
34.4±0.8# |
|
5 |
13.6±0.5 |
13.8±0.5 |
12.8±0.5# |
13.8±0.4 |
13.8±0.7# |
14.4±0.4 |
|
6 a |
12.4±0.2# |
13±0.3 |
13.8±0.3*# |
13.8±0.3*# |
12.8±0.5# |
13.4±0.6 |
|
6 b |
14±0.7 |
12.2±1.9 |
13.6±0.4# |
14±0.3# |
13.4±0.6# |
14±0.6 |
|
7 |
12.6±0.2# |
14.2±0.3* |
13.2±0.5# |
13.8±0.5 |
13.4±0.6# |
14.2±0.755 |
|
8 |
18±0.5*# |
19.6±0.4*# |
21.8±0.3*# |
23±0.5*# |
23.6±0.4*# |
24.4±0.4*# |
|
10 a |
18.6±0.6*# |
23.4±1.1*# |
23.8±0.9*# |
24.8±1*# |
24.8±0.8*# |
25.8±0.5*# |
|
11 |
37±0.8# |
37.4±0.7# |
36.2±0.5# |
36.6±0.6# |
36±0.4# |
36.2±1.4# |
|
12 b |
11.8±0.3# |
12.2±0.7 |
13.8±0.5 |
14±0.4* |
13.4±0.87# |
14±0.7* |
|
12 c |
12.8±0.3 |
13.8±0.5 |
14±0.6 |
13.6±0.6 |
13.8±0.5# |
13.6±0.6 |
|
12 d |
13.8±0.5 |
15.2±1.5 |
14±0.4# |
15.4±0.2* |
15±0.3 |
15.2±0.6 |
|
12 f |
13.8±0.5 |
13.8±0.5 |
13.8±0.5 |
13±0.5# |
14±0.6# |
16±0.4* |
|
12 g |
13.8±0.6 |
14±0.5 |
14.8±0.4 |
14±0.7 |
13±0.7 |
12.4±0.7 |
|
13 |
18.2±1.3# |
20.8±0.7# |
22±0.5*# |
23.8±0.3*# |
23.8±0.4*# |
21.4±0.2*# |
|
14 a |
16±0.5# |
15±0.8 |
15.6±0.5 |
17.4±0.5# |
14.2±0.4# |
14.6±0.4 |
|
14 b |
19.8±0.8# |
21±0.7# |
22±0.8# |
23±0.8*# |
23.6±0.6*# |
20.6±0.4# |
|
15 a |
16±0.6# |
15.4±0.2# |
15.4±0.5 |
14.4±0.2* |
14±0.6# |
14±0.4* |
*Significantly different from 10 min.time point in the same group P<0.05
# Significantly different from the same time point in control group<0.05.
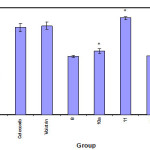 |
Figure 1 Click here to View figure |
Table 3: Anti-inflammatory activity of compounds 5, 6a, b, 7, 8, 10a, 11, 12b-f, 13, 14a,b and 15a.
|
Time |
Thickness |
||||
| Mean±SEM |
Zero time |
1st hr |
2nd hr |
3rd hr |
4th hr |
| Control |
0.31±0.01 |
0.78±0.04* |
0.89±0.03* |
1.16±0.02* |
1.4±0.04* |
| Celecoxib |
0.31±0.01 |
0.38±0.01*# |
0.47±0.01*# |
0.53±0.03*# |
0.63±0.02*# |
| Volatrin |
0.31±0.01 |
0.43±0.03*# |
0.54±0.03*# |
0.57±0.02*# |
0.71±0.03*# |
|
5 |
0.31±0.01 |
0.57±0.02*# |
0.65±0.02*# |
0.69±0.03*# |
0.77±0.03*# |
|
6a |
0.31±0.01 |
0.71±0.04* |
0.85±0.02* |
1.14±0.02* |
1.24±0.02*# |
|
6b |
0.31±0.01 |
0.75±0.02* |
0.80±0.03* |
0.98±0.06*# |
1.24±0.02*# |
|
7 |
0.31±0.01 |
0.82±0.06* |
0.90±0.04* |
1.16±0.02* |
1.26±0.06*# |
|
8 |
0.31±0.01 |
0.86±0.02* |
1.03±0.06* |
1.10±0.05* |
1.20±0.03* |
|
10a |
0.31±0.01 |
0.62±0.02*# |
0.82±0.02* |
0.85±0.02*# |
0.92±0.02*# |
|
11 |
0.31±0.01 |
0.48±0.03*# |
0.47±0.01*# |
0.53±0.03*# |
0.64±0.02*# |
|
12b |
0.31±0.01 |
0.80±0.04* |
0.88±0.02* |
1.1±0.05* |
1.3±0.04* |
|
12c |
0.31±0.01 |
0.75±0.02* |
0.94±0.02* |
1.16±0.02* |
1.4±0.03* |
|
12d |
0.31±0.01 |
0.60±0.03*# |
0.63±0.03*# |
0.72±0.03*# |
0.79±0.01*# |
|
12f |
0.31±0.01 |
0.55±0.02*# |
0.63±0.02*# |
0.71±0.04*# |
0.72±0.03*# |
|
12g |
0.31±0.01 |
0.55±0.03*# |
0.61±0.01*# |
0.65±0.03*# |
0.72±0.02*# |
|
13 |
0.31±0.01 |
0.78±0.02* |
0.94±0.02*# |
1.16±0.02* |
1.36±0.04* |
|
14a |
0.31±0.01 |
0.61±0.02*# |
0.70±0.02*# |
0.77±0.01*# |
0.86±0.02*# |
|
14b |
0.31±0.01 |
0.84±0.04* |
0.88±0.02* |
1.20±0.03* |
1.34±0.04* |
|
15a |
0.31±0.02 |
0.47±0.03*# |
0.55±0.04*# |
0.61±0.05*# |
0.85±0.02*# |
*significantly different from zero time point in the same group P<0.05
# significantly different from the same time point in control group P<0.05.
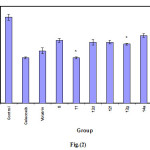 |
Figure 2 Click here to View figure |
Table 4: Ulcerogenic activity of compounds 5, ,8,10a,11,12d-g,13,14a,b and 15a.
|
Group |
score |
|
Mean ± SEM |
|
|
Control |
0.00 ± 0.00 |
|
Celecoxib |
0.00 ± 0.00 |
|
Diclofenac |
4.60 ± 0.24 # |
|
5 |
0.20 ± 0.20 |
|
8 |
0.00 ± 0.00 |
|
10a |
0.20 ± 0.20 |
|
11 |
0.20 ± 0.20 |
|
12d |
2.20 ± 0.37 # |
|
12f |
0.20 ± 0.20 |
|
12g |
0.40 ± 0.24 |
|
13 |
0.20 ± 0.20 |
|
14a |
0.40 ± 0.24 |
|
14b |
0.40 ± 0.24 |
|
15a |
0.40 ± 0.24 |
# significantly different from the control group p< 0.05.
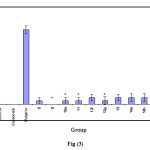 |
Figure 3 Click here to View figure |
A- Chemistry
Melting points were determined with a Gallen Kamp melting point apparatus and are uncorrected. IR spectra (KBr, cm-1) were recorded on Bruker or Testscan Shimadzu FT 8000 spectrometers. 1HNMR ,C13NMR spectra were recorded on Varian Gemini 200, 200 MHz, Varian Mercury (300 MHz) using DMSO-d6 or CDCl3 as solvent and (TMS) as internal stander (chemical shift in d, ppm). Electron impact mass spectra were determined using a GC/MS Mat112 S at 70 eV spectrometer. Elemental analysis were determined using the Elemental analyzer, Heraeus and Automatic Elemental analyzer, Model 2400 Perkin Elemer at Microanalytical Center, Faculty of Science, University of Cairo, Egypt. Thin layer chromatography (TLC) was performed on Silica gel G for TLC (Merck) and spots were visualized by iodine vapors or by irradiation with ulteraviolet light (uv; 254 nm). Compounds 3, 4 were prepared according to reported procedures. (15) Compound (3) m.p 198-200Co, yield 95% as reported IR (KBr, cm-1): 3216 (NH), 3062 (CH, aromatic), 2987, 2937 (CH, aliphatic), 1691 (C=O ester), 1604 (C=N), 1508 (C=C) and 1303 (SO2). Compound (4) m.p. 279-280°C, yield 90% as reported. Microanalysis for C14H15N3O3S (305) Calcd%, C, 55.08; H, 4.9; N, 13.77, Found % C, 54.93; H, 4.59; N, 13.76. IR (KBr, cm-1): 3322 (NH2), 3149 (NH), 3048 (CH, aromatic), 2940 (CH, aliphatic), 1650 (C=O), 1608 (C=N), 1579 (C=C), 1342 (SO2). Ms: m/z (%) 305 (5.21) M+, 290 (0.98), 274 (100), 182 (2.39), 155 (12), 139 (9.20), 119 (33.96), 91 (75.80), 65 (22.98).
N-(2, 5-Dimethyl-1H-pyrrol-1-yl) 4-tosylaminobenzamide(5)
A mixture of the hydrazide 4 (0.610 gm, 0.002 mol) and 2,5-hexanedione (0.228 gm, 0.002 mol) in glacial acetic acid (10 ml) was stirred at room temperature overnight. Dilution with water gave reddish solid was filtered, crystallized from aqueous ethanol yield, 95% ,m.p.148-150Co.Microanalysis for C20H21N3O3S (383.46) Calcd% C, 62.64; H, 5.52; N, 10.96 Found% C, 62.76; H, 5.30; N, 10.76. IR(KBr, cm-1): 3320 (NH), 3193 (NH), 3051 (CH, aromatic), 2922 (CH, aliphatic), 1760 (C=O), 1605 (C=N), 1487 (C=C), 1337(SO2). 1HNMR (DMSO-d6) d ppm: 2.05 (s, 6H, 2CH3), 2.4 (s, 3H, CH3), 5.74 (s, 2H, CH pyrrol) 7.257 (d, 2H J=8.1Hz ArH),7.387(d,2H, J=8.1Hz ArH)7.754(d,2H, J=7.8Hz ArH),7.848(d,2H, J=8.7Hz ArH) 10.80 (s, 1H, NH), 11.13 (s, 1H, NH). 13C-NMR (DMSO-d6) d ppm 11.012, 20.979, 103.067, 118.14, 126.56, 126.72, 128.86, 129.85, 137.6, 141, 143.6, 161.11.
General procedure for the preparation of 6a, b&7: To a solution of the acid hydrazide 4 (10 mmol) in ethanol (30 ml) containing glacial acetic acid( 2 ml )an equimolar amount of acetyl acetone or benzoyl acetone or ethyl acetoacetate was added. The reaction mixture was heated under reflux for 12 hrs, concentrated to a small volume, cooled, and added to ice-cold water(30 ml). The separated product was filtered, washed with H2O and recrystalized from an appropriate solvent.
[(3,5-Dimethyl-1H-pyrazol-1-yl) 4-(tosylamino)] phenyl methanone (6a):
Crystallized from EtOH Yield 75% ,m.p. 238-240°C, Microanalysis for C19H19N3O3S (369.44) Calcd% C, 61.77; H, 5.18; N, 11.37 Found% C, 61.37; H, 5.59%; N, 11.46. IR (KBr, cm-1): 3258 (NH), 3055 (CH, aromatic), 2979, 2930 (CH, aliphatic), 1690 (C=O), 1603 (C=N), 1505 (C=C),and 1336 (SO2). 1HNMR (DMSO-d6). dppm: 1.28 (s, 3H, CH3), 2.16 (s, 3H, CH3), 2.36 (s, 3H, CH3), 6.24 (s, 1H, CH of pyrazol), 7.26-7.82 (m, 8H, ArH), 10.81 (s, 1H, NH).
[(3-Methyl-5-phenyl-1H-pyrazol-1-yl)-4-(tosylamino)] phenyl methamone (6b):
Crystallized from EtOH yield 70%; m.p. 285-290°C . Microanalysis for C24H21N3O3S (431.5) Calcd% C, 66.80; H, 4.91; N, 9.74 Found% C, 66.97; H, 5.48%; N, 9.28. IR (KBr, cm-1): 3195 (NH), 3058 (CH, aromatic), 2930 (CH, aliphatic), 1620 (C=O), 1509 (C=N), 1444 (C=C), 1338 (SO2). 1HNMR (DMSO-d6) dppm 2.03 (s, 3H, CH3), 2.30 (s, 3H, CH3), 5.80 (s, 1H, CH of pyrazol), 7.20-7.95 (m, 13H, ArH), 10.71 (s, 1H, NH).
[(3-Methyl-5-oxo-1H-pyrazolin-1-yl) 4-(tosylamino)] phenyl methanone (7):
Crystallized from EtOH yield 65%; m.p. 195-198°C . Microanalysis for C18H17N3O4S (371.4) Calcd% C, 58.21; H, 4.61; N, 11.31 Found% C, 58.41; H, 4.96%; N, 11.00. IR (KBr, cm-1): 3210 (NH), 3060 (CH, aromatic), 2980, 2937(CH, aliphatic), 1688 (C=O), 1605 (C=N), 1506 (C=C), 1335 (SO2). 1HNMR (DMSO-d6) dppm: 1.28 (s, 3H, CH3), 2.32 (s, 3H, CH3), 4.20 (d,1H, J= 6.6Hz CH2) ,4.24(d,1H, J= 6.6Hz,CH2)7.19(d,2H,J=8.1Hz, ArH), 7.34(d,2H, J=7.8Hz ArH)7.714(d,2H ,J= 7.8Hz,ArH)7,79(d,2H,J= 8.1Hz,ArH) 11.00 (s, 1H, NH).
N-[3, 4, 5-Trimethoxybenzylidene]-4-tosylamino benzohydrazide (8)
A mixture of the hydrazide 4 (0.610gm,0.002mol) and 3,4,5-trimethoxybenzaldehyde (0.392 gm, 0.002 mol) was dissolved in ethanol (15 ml) and heated under reflux for 3 hrs. Cool the mixture the separated solid was filtered washed with water and crystallized from absolute EtOH, yield 90%; m.p. 259-260°C. Microanalysis for C24H25N3O6S (484) Calcd% C, 59.61; H, 5.21; N, 8.69 Found% C, 59.15; H, 5.71%; N, 8.59. IR (KBr, cm-1): 3474 (NH), 3173 (NH), 3050 (CH, aromatic), 2937 (CH, aliphatic), 1645 (C=O), 1607 (C=N), 1574 (C=C), 1329 (SO2). 1HNMR (DMSO-d6) dppm: 2.33 (s, 3H, CH3), 3.69 (s, 3H, OCH3), 3.82 (s,6H,2OCH3),6.99(s,2H,ArH),7.218(d,2H,J=8.7Hz,ArH),7.381(d,2H,J=8.4Hz,ArH)7.70-7.77 (m,4H,ArH) 8.3 (s, 1H, HC=N), 10.69 (s, 1H, NH), 11.69 (s, 1H, NH).
N-(4-Chlorobenzoyl)-4-tosylaminobenzohydrazide (9):
A mixture of the hydrazide 4(0.610 gm, 0.002 mol) and p-chlorobenzoyl chloride(0.348gm,0.002mol) in glacial acetic acid,(10ml) stirred at room temperature for 3 hrs, the separated solid was filtered, washed with water and crystallized from absolute EtOH, yield 80%; m.p. 210-212°C. Microanalysis for C21H18ClN3O4S (444) Calcd% C, 56.82; H, 4.09; N, 9.47 Found% C, 57.50; H, 4.45%; N, 9.56. IR (KBr, cm-1): 3236 (NH), 3007(CH, aromatic), 2843 (CH, aliphatic), 1685 (C=O), 1610 (C=N), 1569 (C=C), 1331 (SO2). 1HNMR (DMSO-d6) dppm: 2.32 (s, 3H, CH3), 7.19-7.97 (m, 12H, ArH), 10.38 (s, 1H, NH), 10.54 (s, 1H, NH), 10.68 (s, 1H, NH),. Ms. m/z (%) 444 (0.27) , 443 (1.13) M+-1, 313 (0.56), 274 (100), 139 (24.2), 119 (16.31), 111 (12.05), 91 (35.47).
General procedure for the preparation of compounds 10a-c. A mixture of acid hydrazide 4 (0.610g, 0.002 mol)and equimolar amount of maleic anhydride or dichloromaleic anhydride or phthalic anhydride in glacial acetic acid(20ml) was heated under reflux for 8 hrs. The product was cooled and then poured into ice water 30 ml. The separated solid was filtered and crystallized from EtOH.
N-[(2, 5-Dioxo-2, 5-dihydropyrrol-1-yl)-4-(tosylamino)] benzamide (10 a):
Yield 75%; m.p. 178-180°C. Microanalysis for C18H15N3O5S (385) Calcd% C, 56.10; H, 3.92; N, 10.90 Found% C, 55.65; H, 4.40; N, 10.68. IR (KBr, cm-1): 3256 (NH), 3005 (CH, aromatic), 2928 (CH, aliphatic), 1735, 1672 (2C=O), 1605 (C=N), 1496. (C=C), 1336. (SO2). 1HNMR (DMSO-d6) dppm: 2.32 (s, 3H, CH3), 7.19 (s, 2H, HC=CH), 7.36-7.70 (m, 8H, ArH), 10.18 (s, 2H, 2NH). Ms. m/z(%) 385 (0.26), 329 (0.61), 305 (1.05), 290 (18.20), 274 (100), 155 (20.35), 119 (33.65), 107 (11.25), 91 (91.14), 65 (20.65).
N-[(3,4-Dichloro-2,5-dioxo-2,5-dihydropyrrol-1-yl)-4-(tosylamino)]benzamide(10b):
Yield 65%; m.p. 168-170°C. Microanalysis for C18H13Cl2N3O5S (454) Calcd% C, 47.59; H, 2.88; N, 9.25 Found% C, 47.40; H, 3.00; N, 8.9. IR (KBr, cm-1): 3243. (NH), 3050 (CH, aromatic), 2926.(CH, aliphatic), 1667 (2C=O), 1606 (C=N), 1498 (C=C), 1335(SO2). Ms. m/z (%) 454 (0.63) , 453 (2.85), 435 (4.78), 426 (2.71), 367 (8.60), 327 (5.41), 191 (26.95), 149 (100), 125 (32.9), 111 (43.81), 85 (35.73), 69 (99.85), 55 (35.14).
N-[(1,3-Dioxoisoindolin-2-yl)-4-(tosylamino)]benzamide(10c):
Yield 85%; m.p. 198-200°C. Microanalysis for C22H17N3O5S (435.5) Calcd% C, 60.68; H, 4.93; N, 9.95 Found% C, 60.23; H, 5.00; N, 10.04. IR (KBr, cm-1): 3424(NH), 3220 (NH), 3060 (CH, aromatic), 2930 (CH, aliphatic), 1737(C=O), 1686 (C=O), 1608 (C=N), 1497 (C=C), 1335 (SO2). Ms. m/z (%) 435 (0.7) , 434 (1.1), 274 (100), 250 (7.6), 120 (13.5), 91 (65.8), 77 (6.9), 51 (9.7).
5-(4-Tosylaminophenyl) 1,3,4-oxadiazole-2-thiol(11)
To a solution of acid hydrazide 4 (0.610gm,0.002 mol) in EtOH(50 ml) (95%) containing KOH (0.130gm,0.0022 mol), CS2 (10 ml )was added. The reaction mixture was heated under reflux for 12 hrs while stirring, then concentrated, cooled and acidified with dilute HCl. The separated product was filtered, washed with H2O and crystalized from aqueous EtOH. yield 95%; m.p. 229-230°C. Microanalysis for C15H13N3O3S2 (347) Calcd% C, 51.86; H, 3.77; N, 12.10 Found% C, 51.74; H, 3.70; N, 12.49. IR (KBr, cm-1): 3278 (2NH), 3056 (CH, aromatic), 2924 (CH, aliphatic), 1508 (C=N), 1470 (C=C), 1344 (SO2). 1HNMR (DMSO-d6) dppm 2.33 (s, 3H, CH3), 7.24-7.73 (m, 8H, ArH), 10.50 (s, 1H, NH), 14.62 (s, 1H, NH). Ms. m/z (%) 347 (63.66), 287 (13.74), 274 (12.99), 192 (32.49), 155 (20.80), 132 (95.80), 91 (100), 78 (10.46), 65 (30.53).
2-(4-Tosylaminophenyl)-5-(alkylthio)-1,3,4-oxadiazoles(12a-d)2-(5-(4-tosylaminophenyl)-1,3,4-oxadiazol-2-ylthio)-N-4(substituted phenyl) acetamides (12e-i)
A mixture of 11 (0.347gm,0.001 mol) and appropriate alkyl or aryl halid or chloroacetamide derivatives(0.001 mol) in ethanol(30ml) containing KOH (0.1gm,0.0017 mol) was stirred at room temperature overnight. The reaction mixture was poured into ice water(50ml). The separated solid was filtered and crystallized from appropriate solvent (Table 5).
Table 5: 2-(4-(Tosylaminophenyl-5-alkylthio)1,3,4-oxadiazoles(12a-d),2-(5-(4-tosylamino phenyl)-1,3,4-oxadiazol-2-yl thio-N-4-(substituted phenyl) acetamides (12e-i).
|
Comp. No |
R2 |
M.P.°C |
Yield % |
Crystalization solvent |
M.F (M.W) |
Analysis (%) calcd/found |
||
|
C |
H |
N |
||||||
|
12a |
CH3– |
122-125 |
85 |
a
|
C16H15N3O3S2 (361.44) |
53.17 53.31 |
4.18 3.89 |
11.63 11.91 |
|
12b |
C2H5– |
198-200 |
75 |
a |
C17H17N3O3S2 (375) |
54.38 54.23 |
4.56 4.33 |
11.19 10.90 |
|
12c |
(CH3)2CH- |
138-140 |
80 |
a |
C18H19N3O3S2 (389.49) |
55.51 55.23 |
4.92 4.61 |
10.79 10.49 |
|
12d |
C6H5-CH2– |
188-190 |
85 |
a |
C22H19N3O3S2 (438) |
60.39 60.16 |
4.38 4.20 |
9.60 9.29 |
|
12e |
C6H5-NH-CO-CH2– |
205-207 |
80 |
b |
C23H20N4O4S2 (480.56) |
57.48 57.63 |
4.19 4.45 |
11.66 11.73 |
|
12f |
4-Cl(C6H4)NH-CO-CH2– |
218-220 |
80 |
b |
C23H19ClN4O4S2 (515) |
53.64 53.35 |
3.72 4.33 |
10.88 11.28 |
|
12g |
4-OCH3(C6H4)NH-CO-CH2– |
208-210 |
85 |
b |
C24H22N4O5S2 (511) |
56.46 56.51 |
4.34 4.52 |
10.97 11.12 |
|
12h |
4-CH3(C6H4)NH-CO-CH2– |
204-206 |
75 |
b |
C24H22N4O4S2 (494.5) |
58.28 58.55 |
4.94 4.59 |
11.33 11.71 |
|
12i |
2-CH3(C6H4)NH-CO-CH2– |
199-201 |
70 |
b |
C24H22N4O4S2 (494.5) |
58.28 58.38 |
4.94 4.91 |
11.33 11.55 |
a = CHCl3/ pet. ether 60-80Co
b= Ethanol
Compound 12a
IR (KBr, cm-1): 3103 (NH), 3008 (CH, aromatic), 2926 (CH, aliphatic), 1608 (C=N), 1469 (C=C), 1340 (SO2), 1HNMR (CDCl3) dppm 2.03 (s, 3H, CH3), 2.73 (s, 3H, CH3, S-CH3), 6.87-7.94 (m, 8H, ArH),10.79(s,1H,NH)
Compound 12b
IR (KBr, cm-1): 3198 (NH), 3058 (CH, aromatic), 2930 (CH, aliphatic), 1477 (C=N), 1404 (C=C), 1335 (SO2), 1HNMR (CDCl3) dppm 1.50 (t, 3H, CH3), 2.38 (s, 3H, CH3), 3.35 (q, 2H, CH2), 7.26-7.73 (m, 8H, ArH),10,97(s,1H,NH).
Compound 12c
IR (KBr, cm-1): 3100 (NH), 3072 (CH, aromatic), 2965, 2921 (CH, aliphatic), 1609 (C=N), 1503 (C=C), 1338 (SO2), 1HNMR (DMSO-d6) dppm 1.438 (d, 6H,J= 6.9Hz, 2CH3), 2.31 (s, 3H, CH3), 3.86-3.88 (m, 1H, CH), 7.301 (d,2H,J=7.2Hz, ArH), 7.371(d,2H,J=7.8Hz,ArH)7.729(d,2H,J= 8.1Hz,ArH)7.845(d,2H,J=8.7Hz,ArH)10.78 (s, 1H, NH). 13CNMR (DMSO-d6) dppm: 20.943, 23.049, 38.39, 127.37, 128.51, 136.16, 137.18, 139.28, 145.8, 150.7, 153.09, 171.9, 174.18.
Compound 12d
IR (KBr, cm-1): 3198 (NH), 3058 (CH, aromatic), 2930 (CH, aliphatic), 1607 (C=N), 1477 (C=C), 1338 (SO2), 1HNMR (CDCl3) dppm: 2.33 (s, 3H, CH3), 4.51 (s, 2H, CH2), 7.26-7.84 (m, 13H, ArH).10.79 (s,1H,NH)
Compound 12e
IR (KBr, cm-1): 3208 (NH), 3073 (CH, aromatic), 2929 (CH, aliphatic), 1665 (C=O), 1608 (C=N), 1337 (SO2), 1HNMR (DMSO-d6) dppm 2.22 (s, 3H, CH3), 4.23(s, 2H, CH2), 7.02-8.23 (m, 13H, ArH), 10.41 (s, 1H, NH), 10.70 (s, 1H, NH).
Compound 12f
IR (KBr, cm-1): 3211 (NH), 3072 (CH, aromatic), 2925 (CH, aliphatic), 1665 (C=O), 1605 (C=N), 1337 (SO2). M.s m/z (%) 515 (0.81) , 498 (0.76), 481 (0.76), 462 (1.21), 452 (1.03), 440 (14.81), 422 (2.19), 367 (5.06), 361 (1.65), 348 (100), 330 (1.47), 3.17 (0.55), 2.87 (39.46).
Compound 12g: IR (KBr, cm-1): 3210 (NH), 3050 (CH, aromatic), 2926 (CH, aliphatic), 1663 (C=O), 1607 (C=N), 1576 (C=C), 1337 (SO2), 1HNMR (DMSO-d6) dppm: 2.33 (s, 3H, CH3),3.73(s,3H,OCH3) 4.26 (s,2H,CH2),7.258(d,2H,J=7.8Hz, ArH),7.374(d,2H,J=7.8Hz,ArH)
7.723(d,4H,J=8.1Hz,ArH) 7.799(d,2H,J=8.1Hz,ArH),7.957(d,2H,J=8.1Hz,ArH),
10.27 (s, 1H, NH), 10.79 (s, 1H, NH).
Compound 12h
IR (KBr, cm-1): 3210 (NH), 3071(CH, aromatic), 2924 (CH, aliphatic), 1665 (C=O), 1605 (C=N), 1545 (C=C), 1336 (SO2), 1HNMR (DMSO-d6) dppm: 2.32 (s, 3H, CH3), 2.42(s,3H,CH3),4.33(s,2H,CH2),7.30-7.76(m,12H,ArH), 10.28 (s, 1H, NH), 10.76(s, 1H, NH).
Compound 12i
IR (KBr, cm-1): 3257 (NH), 3072, 3004 (CH, aromatic), 2928 (CH, aliphatic), 1653 (C=O), 1606 (C=N), 1545 (C=C), 1331 (SO2). 1HNMR (DMSO-d6) dppm: 2.20 (s, 3H, CH3), 2.33 (s, 3H, CH3), 4.23 (s, 2H, CH2), 6.74-7.86 (m, 12H, ArH), 10.69 (s, 1H, NH), 11.21 (s, 1H, NH).
N-[(5,5-Dimethyl-3-oxocyclohex-1-enyl)4-tosylamino] benzohydrazide (13)
A mixture of acid hydrazid 4 (1.2 gm, 0.004 mol) and equimolar amount of 5,5-dimethyl-1,3-cyclohexanedione (dimedone) (0.56 gm, 0.004 mol) in toluene(20 ml) was heated under reflux for 15 hrs. The reaction mixture allow to cool to room temperature. The obtained crystalline product was filtered and recrystallized from ethanol as yellow crystal, yield 75%, m.p. 237-238°C. Microanalysis for C22H25N3O4S (427) Calcd % C, 61.81; H, 5.89; N, 9.83 Found % C, 61.84; H, 5.94; N,. 10.17, IR (KBr, cm-1): 3237 (3NH), 3013 (CH, aromatic), 2951 (CH, aliphatic), 1671 (2C=O), 1549 (C=C), 1331 (SO2). 1HNMR (DMSO-d6) dppm: 0.78 (s, 3H, CH3), 1.00 (s, 3H, CH3), 2.01 (s, 2H, CH2), 2.30 (s, 5H, CH3+ CH2), 4.89 (s, 1H, =CH), 7.14-7.68 (m, 8H, ArH), 9.58 (s, 1H, NH), 10.60 (s, 1H, NH), 11.20 (s, 1H, NH).
N-(2-Amino-3-cyano-7,7-dimethyl-4-(4-substitutedphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinolin-1-yl-4-tosylaminobenzamides 14(a-c)
A mixture of equimolar amounts of enaminone 13 (0.626 gm, 0.001 mol) and the appropriate 2-(4-substituted benzylidene) malonodinitrile (0.001 mol) in absolute ethanol (15 ml) containing TEA (3 drops) was heated under reflux for 12-20 hrs. The reaction mixture was cooled and the separated solid product was filtered, washed with water and crystallized from ethanol (Table 6).
Table 6: N-(2-Amino-3-cyano-7,7-dimethyl-4-(4-substitutedphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinolin-1-yl-4-tosylaminobenzamides 14(a-c).
|
Comp No. |
X |
Time reflux (hrs) |
m.p. °C |
Yield % |
MF (M.W) |
Analysis% Calcd/found |
||
|
C |
H |
N |
||||||
|
14a |
Cl |
15 |
180-182 |
65 |
C32H30ClN5O4S (616.13) |
62.38 62.24 |
5.75 5.38 |
11.37 11.00 |
|
14b |
NO2 |
12 |
218-220 |
70 |
C32H30N6O6S (626.68) |
61.33 61.43 |
4.83 5.43 |
13.41 13.38 |
|
14c |
OCH3 |
20 |
205-208 |
60 |
C33H33N5O5S (611.7) |
64.79 64.71 |
5.44 5.88 |
11.45 11.32 |
Compound (14a)
IR (KBr, cm-1): 3457, 3349 (NH2), 3215, (NH), 3055 (CH, aromatic), 2957 (CH, aliphatic), 2183 (CN), 1647 (C=O), 1486 ( (C=C), 1336 (SO2). Ms: m/z (%) 616 (4.02), 615 (0.15), 597 (50.59), 442 (75.84), 290 (35.32), 274 (48.69), 91 (73.57), 64 (100).
Compound (14b)
IR (KBr, cm-1): 3457, 3351 (NH2), 3205 (NH), 3051 (CH, aromatic), 2952 (CH, aliphatic), 2183(CN), 1632 (C=O), 1512 (C=N), 1459.8 ,1379.8(NO2), 1341 (SO2). 1HNMR (DMSO-d6) d ppm: 0.80 (s, 3H, CH3), 0.85 (s, 3H, CH3), 2.00-2.20 (m, 4H, 2CH2),2.30(s,3H,CH3)4.40(s,1H,CH),6.58(s,2H,NH2,exchangeable),7.0267(d,2H,J=8.4Hz,ArH)),7.0389(d,2H,J=7.8Hz,ArH),7.0661-7.0776(m,4H,ArH)7.0874(d,2H,J=8.4Hz,ArH), 8.0152(d,2H,J=8.7Hz,ArH), 11.00 (broad 2H, 2NH, exchangeable). 13CNMR (DMSO-d6) dppm: 21.02, 27.6, 28.11, 37.1, 49.2, 57.3, 110.9, 117.77, 121.2, 123.4, 126.8, 129.7, 136.6, 142.2, 143.7, 146, 165.7, 194.64.
Compound (14c)
IR (KBr, cm-1): 3374 (NH2), 3238 (NH), 3050 (CH, aromatic), 2952 (CH, aliphatic), 2184 (CN), 1645 (C=O), 1606.(C=N), 1506 (C=C), 1337 (SO2). 1HNMR (DMSO-d6) d ppm: 0.83 (s, 3H, CH3), 0.98 (s, 3H, CH3), 2.00-2.20 (m, 4H, 2CH2), 2.30 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 4.85 (s, 1H, CH), 6.58 (s, 2H, NH2), 7.24-7.76 (m, 12H, ArH), 10.40 (s, 1H, NH), 10.85 (s, 1H, NH).
N-(Ethyl-2-amino-7,7-dimethyl-4-(4-substitutedphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinolin-1-yl-3-carboxylate-4-tosylaminobenzamides (15a-b)
The title compound were prepared as described under 14a-c using the enaminone 13 and ethyl 3-(4-substituted phenyl) 2-cyanoacrylate then heating under reflux for 10-12 hrs, crystallization from ethanol(Table 7).
Table 7: N-(Ethyl-2-amino-7,7-dimethyl-4-(4-substitutedphenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinolin-1-yl-3-carboxylate-4-tosylaminobenzamides (15a-b).
|
Comp No. |
X |
Time reflux (hrs) |
m.p. °C |
Yield % |
MF (M.W) |
Analysis% Calcd/found |
||
|
C |
H |
N |
||||||
|
15a |
Cl |
12 |
270-272 |
65 |
C34H33ClN4O6S (363.18) |
61.58 61.96 |
5.32 5.31 |
8.45 8.80 |
|
15b |
NO2 |
10 |
282-285 |
70 |
C34H35N5O8S (673.22) |
60.61 60.55 |
5.24 5.11 |
10.39 10.54 |
Compound (15a) IR (KBr, cm-1): 3436, 3233 (NH2), 3149 (NH), 3045 (CH, aromatic), 2932 (CH, aliphatic), 1692 (C=O), 1604 (C=N), 1503 (C=C), 1333 (SO2). M.S: m/z (%) 663 (5.62) , 662 (8.08), 597 (15.20), 436 (22.74), 422 (26.11), 369 (50.78), 257 (20.44),. 208 (23.86), 185 (21.08), 173 (18.41), 147 (35.42), 127 (28.52), 96 (53.50), 73 (70.3).
Compound (15b) IR (KBr, cm-1): 3492, 3322 (NH2), 3181 (NH), 3050 (CH, aromatic), 1925 (CH, aliphatic), 1664(C=O), 1621 (C=O), 1507,1379.8 (NO2), 1339 (SO2). 1HNMR (DMSO-d6) d ppm: 0.76 (s, 3H, CH3), 0.95 (s, 3H, CH3), 1.08 (t, 3H, CH3), 1.9-2.1 (m, 4H, 2CH2), 2.34 (s, 3H, CH3)3.91 (d, 2H,J=6.9Hz,CH2), 4.86(s,1H,CH), 7.24 (d,2H,J=8.7Hz,ArH), 7.37 (d,2H,J=7.8Hz,ArH),7.45(brs,2H,NH2), 7.74 (d,2H,J=8.1Hz,ArH) ,7.91 (d,2H,J=8.1Hz,ArH), 8.03 (d,2H,J=8.4Hz,ArH), 8.09 (d,2H,J=9Hz,ArH)10.80(s, 1H, NH), 11.06(s, 1H, NH).
B- Biological studies
1- Antimicrobial activity
Different bacteria and fungi were subjected to susceptibility test on Muller-Hinton agar medium by cup plate method(16-17). The antimicrobial activity of some of the newly synthesized compounds was tested against each of the mentioned strains using Cefotaxime, Sulphamethoxazole and Nystatin as reference (table 1). Control testing using DMF was used to determine the solvent effect.
Overnight culture was streaked on the cups of Muller-Hinton agar plate with 50 mg/ml concentration of each sample under investigation in DMF solvent the plate incubated at 37°C for 24 hrs and examined for inhibition zones to determine the activity of tested compounds
2- Pharmacological activities:
Analgesic activity evaluation
The hot plate method of Jacob and Bosovski(18)was used to evaluate the analgesic activity. Mature albino mice of both sex weighing 20-25 gm were classified into 19 groups (each of 5). The first group was left as control and injected i.p. with the solvent(DMSO).While the second Group was injected with Celecoxib and Voltarin at a dose of 1.7 mg/kg. Each of the remaining groups was Injected i.p with a test compound at adose of 1.7mg/kg.Ten minutes latter, each mouse was placed in two liter-beaker immersed in water bath thermostatically controlled at 56Co .The time elapsed till the mouse licks its paw or jumps was considered the reaction time and was taken as a measure of the analgesic effect. Reading were taken at 10,20,30,60,90 and 120 minutes post treatment (Table2)
Anti-inflammatory activity evaluation
The rat hind paw odema method(19-21)was applied to determined the Anti-inflammatory activity of the test compounds using Celecoxib ,Voltarin as standerd.Mature albino rats of both sex weighing 200-250gm were used the animal were divided into 19 equal groups (each of 5). The first group was left as control, while the second and third groups was injected(i.p) with Celecoxib and Voltarin at a dose 18 mg/kg- one hour later odema in the right hind paw was induced by injection of 0.1cm3 of 10% carrageenin. The thickness of the paw was measured 60,120,180 and 240 minutes after carrageenin injection to determine the anti-inflammatory activity of the test compounds(Table3)
Ulcerogenic activity(22)
Compounds 5,6a ,8,10a,11,12d,12f,12g,14a,14b and15a were tested for their ulcerogenic activity using Celecoxib and Indomethacin as refrence drug. Male albino rats weighing 150-200gm were fasted for 12hrs prior to drug administration. Water was given ad-libitum.The animal were divided into 15 equal groups(each4).The first group received1% gum acacia(suspending vehicle) orally once a day and was left as control. While the second and third groups received Indomethacin and Celecoxib at a dose 18 mg /kg/day orally.the remaining groups received the test compounds at a dose of 18 mg /kg/day orally. The drugs were administrated once a day for successive days .The animal were killed by an over dosage of ether 6hrs after the last dose .The stomach was removed .Open along the greater curvature and examined ulceration. The number and the severity of discrete areas of damage in the glandular mucosa were scored (Table 4)
Acknowledgement
I am heart fully thankful to extent my profound gratitude to Ass. Lecture Nader Shawky Mohammed ,Microbiology department, Faculty of pharmacy, Zagazig university for his kind support for performing antimicrobial activity.
My extreme thanks and deep gratitude to Prof. Dr. Saed Abdel Aziz, Professor of pharmacology ,Faculty of Veterinary Medicine,Zagazig university for his valuable efforts in carrying the analgesic, anti-inflammatory and ulcerogenic testing.
References
- Ozbek, N.; Katircioglu, H.; Karacan, N. And Baykal, T. Bioorg.& Med.Chem.(2007);15: 5105-5109 .
- Mahantesha, B.K.; Manohar, V.K.; Vijaykumar, P.R.; Harishchandra, P.; Sumit, S.M. and Ashwini, A.M. Eupr.J. of Med. Chem.(2010)45 (3): 1151-1157 .
- Mogilaiah, K.& Vidya, K. Ind. J. Med. Chem. (2006) 45B, 1905 .
- Yogesh, M.; Vaibhav, M. and Devender, P. Int. J. of Drug Design and Discovery 2(2011) (4), 659-665 .
- Somani, R.R.; Shirodkar, P.Y.; Toraskar, M.P. and Kadam, V.J. Ind. J. Pharm. Educ. Res. (2008) 42-53 .
- Jayashankar, B.; lokanath Rai, K.M.; Baskaran, N. and Sathish, H.S. Eur. J. Med. Chem. (2009). 44, 3898 .
- Ullas P.D.; Revanasiddappa, B.C. and Subrahmanyam, E. Int. J of Pharm. Chem. And Biological Sciences(2012) 2 (3). 202-205 .
- Jain, K.; Bhardwaj, S.; Parashar, N. and Sharma, V.K. Int. J. Biopharm. (2001) 2 (1). 14 .
- Mostafa, M.G.; Helmy, I.H.; Amina, A.H.; Amany, B.A. and Marwa, G.E. J. of American Sceince, (2011) 7 (1)1063-1073.
- Eatedal, H.A.; Osama, I.E.; Shaker, Y. and Sameh, M.E. Monatshefte fur Chemie(2002) 133, 255-266 .
- Bhaskar, S.D.; Santosh, S.C.; Gajanan, G.M.; Basseer, M.S.; Santosh. N.K. and Sujata, R.C. Arch. of Applied Sceicne Research(2011) 3 (1), 401-406 .
- Hesse,E Arch. Exp. Pathol. (1954) 110, 130 .
- Savini, L.; Chiasserinl, C.; Pellerano, W. and Filippelli, G. Falcone Farmaco. (2001) 56, 939 .
- Mohammad, A. and Agarwal, R. Ind. J. of Heterocyclic Chem. (1998) 8,225.
- Enany, M.M.; El-Kerdawy, M.M.; Abou Ouf, A.A. and Abou Zeied, Y.M., U.A. R Journal of Pharmaceutical Sceinces(1971) 12 (1), 17-23 .
- Saundane A R. Rudresh K.Sathyanarayana N D and Hiremath S P,Indian J Pharm Sci.1998,60,397.
- Barry A L,the antimicrobial susceptibility test:principle and practice(Illus Lea and Febiger,Philadelphia)1976,180.Biol Abstr,64,1977,25183.
- Jacob J, Bosovski, M(1965) In: Turner RA (ed) Screening methods in pharmacology. Academic Press, New York, London, p104.
- Winter, C A; Filsley E A& Nuss CM, Proc. Soc. Exp. Biols(New York) , 544(1962)111.
- Sirodia P & Rao. Symposium (CNS Drugs), Regional Research Laboratory, Hydrabad,1,1966, 238.
- Maria K; Dimitra HL. and Maria G.J. Med. Chem. (2008)4, 586-596
- Pauls F., Wick AN, Mackay EM(1947)Gastroenterology8:774

This work is licensed under a Creative Commons Attribution 4.0 International License.









