A Whole-cell Biocatalysis Application of Steroidal Drugs
Syed Adnan Ali Shah 1,2*, Sadia Sultan ultanultan 1,2 and Humera Syed Adnan 3
1Department of Pharmacology and Chemistry, Faculty of Pharmacy, Universiti Teknologi MARA (UiTM) Puncak Alam Campus, 42300 Bandar Puncak Alam, Selangor Darul Ehsan, Malaysia.
2Atta-ur-Rahman Institute for Natural Products Discovery, Universiti Teknologi MARA (UiTM) Puncak Alam Campus, 42300 Bandar Puncak Alam, Selangor Darul Ehsan, Malaysia.
3H.E.J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences (ICCBS), University of Karachi, Karachi, Pakistan.
CorrespondingAuthor E-mail: syedadnan@salam.uitm.edu.my
DOI : http://dx.doi.org/10.13005/ojc/290201
Article Received on : April 10, 2013
Article Accepted on : May 27, 2013
Biocatalysis is also widely applicable for enhancement of bioprocess productivity, selectivity of target reactions and production of valuable pharmaceutical ingredients, precursors and key intermediates. Microorganisms and cell suspension cultures of plant have found wide application in biotransformations of steroidal drugs in order to obtain high regio- and stereoselective products. Studies of steroid modifications catalyzed by microbial or plant cell cultures represent a well-established research approach and methodology in white biotechnology. Bioconversion can be done at the position of steroid molecule hardly available for chemical agents; functionalization of the molecule can be performed stereospecifically; several reactions can be completed in one biotechnological step.
KEYWORDS:Steroidal drugs; Microbial transformation; Hydroxylation; Whole-cell biocatalysis; Plant cell cultures
Download this article as:| Copy the following to cite this article: Shah S. A. A, ultanultan S. S, Adnan H. S. A Whole-cell Biocatalysis Application of Steroidal Drugs. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Shah S. A. A, ultanultan S. S, Adnan H. S. A Whole-cell Biocatalysis Application of Steroidal Drugs. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22040 |
Introduction
Biocatalysis is an increaingly important tool in organic synthesis. Biotransformations with whole cells are extensively employed as an industrial method to synthesize many steroidal drugs and chiral building blocks, and to modify natural products with potent bilogical activities. Such studies are primarily useful in the generation of hydoxylated metabolites for drug toxicity studies. Microorganisms such as fungi, bacteria and yeast possess multi-enzyme systems which have significant regio- and stereo-selectivity. Thus, microbial transformation technique is increasingly being used as one of the feasible approaches by far for structurally modifying natural and synthetic compounds. Microbial hydroxylation of steroid is industrially important since it involves the synthesis of many intermediates necessary for the synthesis of many steroidal drugs1-2.
Microbial transformation has also found wide applications in biotransformation of steroids in order to obtain high regio- and stereoselective products. They have been utilized successfully as in vitro models to mimic and predicts the metabolic fate of drugs and other xenobiotics in mammalian systems. They can transform fine chemicals extensively through environmental friendly and cost effective way. The transformat ion mostly carried out by fungi included hydroxylation, oxidation and reduction of alcohols, ketones and C=C bond2-3.
Steroids (Greek, stereos = solids) are solid alcohols that are widely distributed in the animal and plant kingdom. The basic skeleton of steroids consists of 17 carbon atoms arranged in the form of a perhydrocyclopentanophenanthrene (Fig. 1). They include great variations in structure and encompass compounds of vital importance to life, such as cholesterol, the bile acids, sex hormones, vitamin D, corticoid hormones, cardiac aglycones, antibiotics, and insect molting hormones. Some of the most potent toxins are steroidal alkaloids. Steroidal compounds are responsible for various important biological functions in the cells, e.g. steroids of androstane, pregnane and estrane series exhibit various hormonal activities; bile acids are essential for lipid digestion and absorption and cardiac aglycones are used for heart failure treatment. Sterols are important constituents of cell membranes, essential for their stability, cell growth, and proliferation and are precursors of bile acids and hormonal steroids. Some steroidal compounds are used as an important source of raw materials for the production of steroids of the androstane, pregnane and estrane series. Totally, about 300 approved steroid drugs are known to date, and this number tends to grow. Steroid pharmaceuticals are ranked among the most marketed medical products and represent the second large category next to antibiotics. The annual production of steroid drugs exceeded 1,000,000 tons and the global market is around US$ 10 billion4-5.
There is a well-known example that chemical steroid synthesis involves 31 reaction steps to yield 1 g cortisone from 615 g of deoxycholic acid (purified from beef bile). Transformation of simple natural precursor molecule using Rhizopus arrhizus ATCC 11145 and Aspergillus niger ATCC 9142 brought down cortisone manufacturing costs from $ 200 (1949) to $ 1 (1979) and reduced 31 chemical steps to 11 steps. Basically, microbial transformations require mild conditions which are of importance when working with molecules unstable at high temperatures or extremal pH values. There is a possibility of easier isomer separation in bioconversion. And, in general, biotechnologies are more ecologically friendly as compared with chemical synthesis6-7.
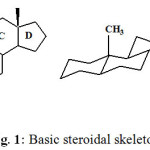 |
Figuire 1: Basic steroidal skeleton. Click here to View table |
Microbial catalysis was established to be the most efficient way to solve another troublesome task for steroid chemistry, which is oxidation of inactivated carbon. Microorganisms such as fungi and bacteria are capable of introducing one or more hydroxyl groups at different positions of the gonane core or side chain are known. Hydroxylation is one of the most important reactions of steroid functionalization. Hydroxylated steroids often express a higher biological activity as compared with their less polar non-hydroxylated analogs. For example, 7α-hydroxy derivatives of DHEA exhibited severalfold higher immunoprotective and immunoregulatory properties as compared with non-hydroxylated DHEA; a few more hydroxylated derivatives of ethisterone displayed tyrosinase inhibitory effects; some novel hydroxy derivatives of HMPD (20-hydroxymethylpregna-1,4-diene-3-one) expressed cytotoxicity towards HeLa cancer lines unlike non-hydroxylated substrate6-8.
The research efforts in microbial technology were triggered around 1950, with the announcement of the pharmacological effects of cortisol and progesterone and with the identification of 11a-hydroxylation activity of a Rhizopus species, a decisive step in the development of the practical synthesis of steroids with useful biological activity. The great versatility of microbial systems in the production of commercially valuable steroids for the pharmaceutical industry is now well recognized. The areas which are now receiving attention in microbial technology are: application of newer concepts of genetic engineering of microbes with improved characteristics, solubility improvement for carrying out biotransformation of substrates that are insoluble in water, immobilization of enzymes or whole cells in a suitable matrix for economic utilization, development of a continuous process for better and economic product recovery; and manipulation of culture media for improvement in product yields by use of cyclodextrin. Several biotransformation of steroidal drugs through microorgainsms and plant cell cultures have been reported3.
During recent years our research has been focused on the structural modifications of bioactive steroids by using various microorganisms and plant cell cultures, in order to obtain biologically potent compounds with diverse structures and various US patents have also been granted on the methods of producing bioactive metabolites through fermentation22-30.
Biotransformation of Phytosterols
Phytosterols, together with sapogenins such as diosgenin, hecogenin and solasodine, are established in the pharmaceutical industry as starting materials for the production of steroidal hormones. It is a well-known fact that a wide range of microorganisms is capable of utilizing sterols such as cholesterol and phytosterols as the sole carbon source for the growth and propagation. It is also well documented that in the sterol degrading microorganisms, the ring nucleus and the side chain are metabolized by different enzyme systems simultaneously and independently9.
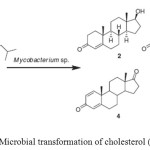 |
Figure 2: Microbial transformation of cholesterol (1). Click here to View figure |
Cholesterol (1) is known as animal sterol; sitosterol, stigmasterol, campesterol, and brassicosterol are abundant plant sterols; the so-called phytosterols are mixtures of plant sterols mainly of soya origin, or produced from tall oil or pitch; ergosterol is a major sterol of yeasts and fungi. Since the 1980s, microbial transformation of phytosterol remains a focus of research in the field of steroids. The production of testosterone (2), a male sex hormone or androgen, from cholesterol (1) via a single step microbial transformation process by Mycobacterium sp. NRRL B-3805. While incubation ofcholesterol (1) with free cells of Arthrobacter simplex afforded cholest-4-en-3-one (3), androst-1, 4-dien-3-17-dione (ADD) (4) and androst-4-en-3,17-dione (5) in an aqueous organic solvent two-phase system (Fig. 2)9-11.
Biotransformation of Steroidal Drugs
Sex hormones are hormones that affects the reproductive system. They include estrogens, progesterone and androgens (such as testosterone). Androgen is the generic term for any natural or synthetic compound, usually a steroidal hormone, that stimulates or controls the development and maintenance of masculine characteristics in vertebrates. Steroid hormones are also known to control distinct aspects of cell proliferation and tissue differentiation and to regulate signal transduction pathways by binding to respective intracellular receptors that act as transcription factors to modulate specific gene expression
C-3 glycoside derivatives of 14-hydroxy-5b, 14b-pregnane show strong interaction with cardiac glycoside receptor of heart muscle. Such compounds have been mainly obtained by multistep chemical transformations of digitoxine, digitoxigenin and related compounds that contain the 5b, 14b-configuration (Fig. 3). Conversely, the 14a-hydroxylation of progesterone (6) and other steroids by several microorganisms is a well documented fact and several fungi have been shown to introduce, in reasonable yields, a hydroxyl group at the 14a-position of progesterone and some other steroids; the 14a-hydroxy configuration can then be transformed chemically to the 14b-hydroxy substituent normally found in cardioactive steroids. Microbial transformation of progesterone (6) by Thamnostylum piriforme yielded 14a-hydroxyprogesterone (7) and 9a-hydroxyprogesterone (8). A transformation with Mucor griseocyanus afforded 7, 6b,14a-dihydroxyprogesterone (9) and 7a,14a-dihydroxyprogesterone (10). The filamentous fungus, Aspergillus fumigatous efficiently hydroxylated 5 producing 11a-hydroxyprogesterone (11), 15b-hydroxyprogesterone (12), 7b-hydroxyprogesterone (13), 7b,15b-dihydroxyprogesterone (14) and 11a,15b-dihydroxyprogesterone (15). Incubation of progesterone (5) with Saprolegnia hypogyna yielded 4-androstene-3, 17-dione (16), testosterone (2) and testololactone (17). Biotransformation of 5 with Fusarium culmorum culture yielded a mixture of 15a-hydroxyprogesterone (18) and 12b,15a-dihydroxyprogesterone (19). However when 5 was fermented with thermophilic bacterium, Bacillus stearothermophilus, three monohydroxylated metabolites and a new metabolite, were isolated and identified. These were identified as 20a-hydroxyprogesterone (20), 6b-hydroxyprogesterone (21), 6a-hydroxyprogesterone (22) and 9, 10-sco-4-pregnene-3, 9, 20-trione (23) as shown in Figure 42-3, 12-14.
Pregnenolone (24) is a major hormone in human nerve tissues and its therapeutic role in repairing defective neurons is well documented. Moreover, it is a precursor of many steroidal hormones. Biological transformation of pregnenolone (24) by Mucor piriformis resulted in the isolation of two metabolites, 3b,7a-dihydroxypregn-5-en-20-one (25) and 3b,7a,11a-trihydroxypregn- 5-en-20-one (26). Fermentation of 24 with the fungus Botrytis cinerea yielded two new oxidized metabolites of pregnenolone. These were identified as 3b,11a,16b-trihydroxypregn-5-en-20-one (27) and 11a,16b-dihydroxypregn-4-en-3, 20-dione (28). Metabolism of pregnenolone (24) by Bacillus strains afforded 25,3b,7b-dihydroxypregn-5-en-20-one (29) and 7-oxopregnenolone (30) as the major metabolites (Fig. 5)2-3.
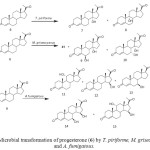 |
Figure 3: Microbial transformation of progesterone (6) by T. piriforme, M. griseocyanus and A. fumigatous. Click here to View figure |
Dehydroepiandrosterone (DHEA) (31) is the most dominant hormone in the body. It is the source of all sex and steroid hormones and known as the body’s mother hormone. Biotransformation of dehydroepiandrosterone (DHEA) (31) was carried out by a plant pathogen Rhizopus stolonifer, which resulted in the production of seven metabolites. These metabolites were identified as 3b,17b-dihydroxyandrost-5-ene (32), 3b,17b-dihydroxyandrost-4-ene (33), 17b-hydroxyandrost-4-ene-3-one (34), 3b,11b-dihydroxyandrost-4-ene-17-one (35), 3b,7a-dihydroandrost-5-ene-17-one (36), 3b,7a,17b-trihydroxyandrost- 5-ene (37) and 11b-hydroxyandrost-4,6-diene-3,17-dione (38) (Fig. 6)15.
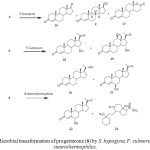 |
Figure 4: Microbial transformation of progesterone (6) by S. hypogyna, F. culmorum and B. stearothermophilus. Click here to View figure |
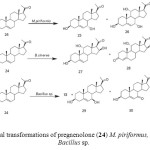 |
Figure 5: Microbial transformations of pregnenolone (24) M. piriformus, B. cinerea and Bacillus sp. Click here to View figure |
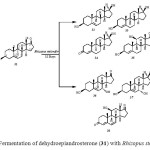 |
Figure 6: Fermentation of dehydroepiandrosterone (31) with Rhizopus stolonifer. Click here to View figure |
Mesterolone ((1a, 5a, 17b)-17-hydroxy-1-methylandrostan-3- one) (39) is a steroidal drug used in the treatment of conditions caused by deficient endogenous androgen formation, which can develop gradually with increasing age. Microbial transformations of mesterolone (39) has been investigated using Cephalosporium aphidicola, Fusarium lini and Rhizopus stolonifer. Metabolites (1a,5a)-1-methylandrostane-3, 17-dione (40), (1a,3b,5a,17b)-1-methylandrostane-3, 17-diol (41) and (1a,5a,15a)-15-hydroxy-1-methylandrostane-3,17- dione (43) were produced by Cephalosporium aphidicola, while metabolites, (5a)-1-methylandrost-1-en-3,17-dione (42), 43, (1a,5a,6a,17b )-6,17-dihydroxy-1-methylandrostan-3-one (44), (1a,5a,15a,17b)-15,17-dihydroxy-1-methylandrostan-3- one (45) and (5a,15a,17b)-15,17-dihydroxy-1-methylandrost- 1-en-3-one (46) were produced by Fusarium lini. Similarly, Rhizopus stolonifer yielded metabolites 40, 44, (1a,5a,7a,17b)-7,17-dihydroxy-1-methylandrostan-3-one (47), (1a,5a,11a,17b)-11,17-dihydroxy-1-methylandrostan-3-one (48) and 46. These transformations are shown in Figure 716.
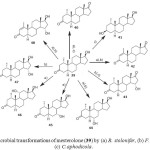 |
Figure 7: Microbial transformations of mesterolone (39) by (a) R. stolonifer, (b) F. lini, and (c) C.aphodicola. Click here to View figure |
Danazol (17b-hydroxy-17a-pregna-2,4-dien-20-yno-[2,3-d] isoxazole (49) is an orally effective, pituitary gonadotropin inhibitor which is effectively used in the treatment of endometriosis, benign fibrocystic mastitis and precocious puberty. Biotransformation of danazol (49) has been carried out using four different fungi and bacteria to study the transformation of danazol (49). Fermentation of danazol (17b-hydroxy-17a-pregna-2,4-dien-20-yno-[2,3-d] isoxazole (49) with Fusarium lini, Aspergillus niger and Cephalosporium aphidicola afforded 17b-hydroxy-2-(hydroxymethyl)-17a-pregn- 4-en-20-yn-3-one (50) and 17b-hydroxy-2-(hydroxymethyl)-17a-pregn-1,4-dien-20-yn-3-one (51), while the fermentation of 49 with Bacillus cerus yielded the metabolite 50 only as shown in Figure 82.
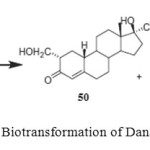 |
Figure 8: Biotransformation of Danazol (49). Click here to View figure |
Prednisone (17a,21-dihydroxypregna-1,4-diene-3,11,20-trione) (52) is commonly used in the treatment of asthma, rheumatic disorders, renal disorders and diseases of inflammatory bowel, skin. Gastrointestinal tract. Fungal transformation of prednisone (52) by Cunninghamella elegans afforded two metabolites, 17a,21-dihydroxy-5a-pregn-1-ene-3,11, 20-trione (53) and 17a,20S,21-trihydroxy-5a-pregn-1-ene-3, 11-dione (54), while fermentation of 52 by Fusarium lini, Rhizopus stolonifer and Curvularia lunata yielded a metabolite 1,4-pregnadiene-17a, 20S, 21-triol-3,11-dione (55) (Fig. 9)2.
Cortisol (56) is an important anabolic hormone clinically used in topical anti-inflammatory and antiallergic medicines. It is also used in the synthesis of many steroidal hormones. Microbial transformation of cortisol (56) with Gibberella fujikuruoi yielded 11b-hydroxyandrost-4-en-3,17-dione (57), while incubation with Bacillus subtilis and Rhizopus stolonifer afforded 11b,17a,20,21- tetrahydroxy- (20S)-pregnt-4-en-3-one (58). The metabolites 11b,17a,21-trihydroxy-5a-pregnan-3, 20-dione (59) and 3b,11b,17a,21-tetrahydroxy-5a-pregnan-20-one (60) were obtained by fermentation of 56 with Bacillus cerus as shown in Figure 102.
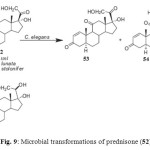 |
Figure 9: Microbial transformations of prednisone (52). Click here to View figure |
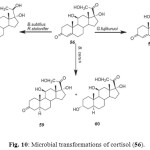 |
Figure 10: Microbial transformations of cortisol (56). Click here to View figure |
Testosterone (17b-hydroxyandrost-4-en-3-one) (2) is a male sex hormone responsible for the development of secondary male characteristics. The hydroxylation of steroids is important because of its physiological role in mammalian organisms and also for practical reasons. Testosterone (2) was actively transformed by M. griseocyanus and T. piriforme. M. griseocyanus produced a major metabolite, 14a,17b -dihydroxyandrost-4-en-3-one (61) and 14a-hydroxyandrost-4-en-3, 17-dione (62). Conversely, T. piriforme produced lower amounts of 14a hydroxylated derivatives and yielded 6b,17b-dihydroxyandrost-4-en-3-one (63), 9a,17b-dihydroxyandrost-4-en-3-one (64) and 6bhydroxyandrost-4-en-3, 17-dione (65). Microbial transformationof testosterone (2) by Nectria haematococca afforded 1-enetestosterone (66) and 11a-hydroxy-1-ene-testosterone (67) (Fig. 11). Fermentation of 2 with Fusarium culmorum yielded 63 and 15a-hydroxytestosterone (68) as the major metabolites. The fungus Cephalosporium aphidicola hydroxylated testosterone (2) to metabolites 61 and 63. The metabolites 63, 68, androst-4-ene-3, 17-dione (69) and 15a-hydroxyandrost-4-ene-3,17-dione (70) were obtained when compound 2 was metabolized with the fungus F. oxysporum var. cubense, while the fungi Curvularia lunata, Pleurotus oestreatus yielded only the metabolites 69 and 70. The plant pathogenic fungus Botrytis cinerea yielded only the metabolite 7b,17b-dihydroxyandrost-3-one (71). Biotransformation of testosterone (2) by Bacillus stearothermophilus resulted in the formation of 63, 68, 6a,17b-dihydroxyandrost-4-en-3-one (72), 6b-hydroxyandrost-4-en-3, 17-dione (73) and 6a-hydroxyandrost-4-en-3, 17-dione (74). These fermented products are shown in Figure 122.
Tibolone (75), a synthetic steroid that is used for hormone replacemen t in menopausal women, is metabolized to three primary active agents: 3a-hydroxytibol one, 3b-hydroxytibolone and D4-tibolone (76). It can exhibit estrogenic, progestogenic or androgenic activity, depending primarily on the target tissue involved; for this reason, tibolone is said to have tissue-specific activity and structurally related to 19-nortestoster one derivatives. Tibolone (75) and its metabolites also showed tissue specific inhibition of sulfatase enzyme which could be protective against development of mammary carcinomas, whereas it retains favorable estrogeni c effects on bone. Whole cells based biotransformation of tissue-specific hormone tibolone (75) was carried out with Trichothecium roseum (ATCC 13411) yielded D4-Tibolone (76), D1,4-tibolone (77), 15a-hydroxy-D4 tibolone (78), 12b-hydroxy-D4-tibolone (79) and 3a,6a-dihydroxy-D4-tibolone (80). These metabolized products are shown in Figure 1314,17.
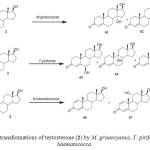 |
Figure 11: Biotransformations of testosterone (2) by M. griseocyanus, T. piriforme and N. haematococca. Click here to View figure |
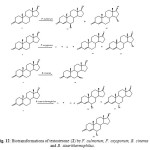 |
Figure 12: Biotransformations of testosterone (2) by F. culmorum, F. oxysporum, B. cinerea and B. stearithermophilus. Click here to View figure |
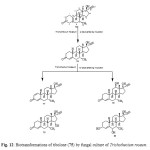 |
Figure 12: Biotransformations of tibolone (75) by fungal culture of Trichothecium roseum. Click here to View figure |
Ethynodiol diacetate (81) is a semi synthetic steroidal drug, used as an oral contraceptive. It inhibits the ovulation process, and serves as a potent progestin. It provides adequate control of menstrual cyclicity in combination with an estrogen, and thus has a complete contraceptive property, even in low doses. Biotransformation of an oral contraceptive, ethynodiol diacetate (81), by using microbial and plant cell cultures provides an efficient route to the synthesis of a library of new steroids with potential contraceptive properties. These methods can be employed in the production of such compounds with high stereoselectivity2.
The biological transformation of ethynodiol diacetate (81) with Cunninghamella elegans and plant cell suspension cultures of Ocimum basilicum and Azadirachta indica was carried out. Biotransformation of 81 with Cunninghamella elegans yielded three new hydroxylated compounds, characterized as 17α-ethynylestr-4-en-3β,17β-diacetoxy-6α-ol (82), 17α-ethynylestr-4-en-3β,17β-diacetoxy-6β-ol (83), and 17α-ethynylestr-4-en-3β,17β-diacetoxy-10β-ol (84) and 17α-ethynyl-17β-acetoxyestr-4-en-3-one (85) as described in Figure 13.
The biotransformation of 81 with Ocimum basilicum included hydrolysis of the ester group, oxidation of alcohol into ketone, and rearrangement of the hydroxyl group. Thus four major metabolites were characterized as 17α-ethynyl-17β-acetoxyestr-4-en-3-one (85), 17α-ethynyl-17β-hydroxyestr-4-en-3-one (86), 17α-ethynyl-3β-hydroxy-17β-acetoxyestr-4-ene (87) and 17α-ethynyl-5α,17β-dihydroxyestr-3-ene (88). Biotransformation of 81 with Azadirachta indica culture yielded compounds 85 and 86 as described in Figure 142.
Exemstane (89: trade name aromasin) is a steroidal irreversible aromatase inhibitor, used for the treatment of breast cancer. Microbial transformation of anti-cancer steroid, exemestane (89), was investigated by using two filamentous fungi, Macrophomina phaseolina, and Fusarium lini. Fermentation of 89 with fungi afforded four polar hydroxyl metabolites, 11α-hydroxy-6-methylene-androsta-1,4-diene-3,17-dione (90), 16β,17β-dihydroxy-6-methylene-androsta-1, 4-diene-3-one (91), and 17β-hydroxy-6-methylene-androsta-1, 4-diene-3, 16-dione (92) and 17β-hydroxy-6-methylene-androsta-1,4-diene-3-one (93) as described in Figure 15 and 1618.
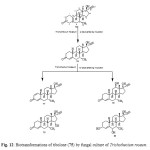 |
Figure 13: Biotransformation of ethynodiol diacetate (81) with Cunninghamella elegans. Click here to View figure |
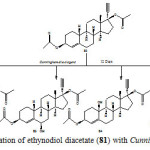 |
Figure 14: Biotransformation of ethynodiol diacetate (81) with cell suspension cultures of Ocimum basilicum (compounds 85–88, in 20 days) and Azadirachta indica (compounds 85 and 86, in 10 days). Click here to View figure |
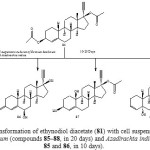 |
Figure 15: Biotransformation of exemestane (89) with Fusarium lini yielded metabolite 90. Click here to View figure |
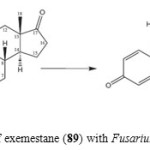 |
Figure 16: Biotransformation of exemestane (89) with Macrophomina phaseolina yielded metabolites 91-93. Click here to View figure |
Microbial transformation of methyloestrenolone (94), an oral contraceptive was investigated by using Macrophomina phaseolina, Aspergillus niger, Gibberella fujikuroi, and Cunninghamella echinulata produced eleven metabolites 95–105. Metabolites were resulted from the hydroxylation at various positions such as C-1, C-2, C-6, C-10, C-11, and C-17a-CH3, as well as aromatization of ring A of the steroidal skeleton of substrate 94. The transformed products were identified as 17a-methyl-6b,17b-dihydroxyestr-4-en-3-one (95), 17a-(hydroxymethyl)-11b,17b-dihydroxyestr-4-en-3-one (96), 17a-methyl-2a,11b,17b-trihydroxyestr-4-en-3-one (97), 17a-methyl-1b,17b-dihydroxyestr-4-en-3-one (98), 17a-methyl-11a,17b-dihydroxyestr-4-en-3-one (99), 17a-methyl-11b,17b dihydroxyestr-4-en-3-one (100), 17a-methyl-10b,17b-dihydroxyestr-4-en-3-one (101), 17a-(hydroxymethyl)-17b-hydroxyestr-4-en-3-one (102), 17a-methylestr-1,3,5(10)-trien-3,17b-diol (103), 17a-methyl-3,17b-dihydroxyestr-1,3,5(10)-trien-6-one (104), and 17a-methyl 6b,10b,17b-trihydroxyestr- 4-en-3-one (105) as described in Figure 17, 18, 19 and 2019.
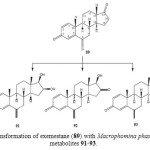 |
Figure 17: Biotransformation of methyloestrenolone (94) with Macrophomina phaseolina. Click here to View figure |
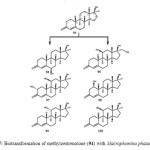 |
Figure 18: Biotransformation of methyloestrenolone (94) with Aspergillus nige. Click here to View figure |
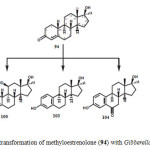 |
Figure 19: Biotransformation of methyloestrenolone (94) with Gibberella fujikuroi. Click here to View figure |
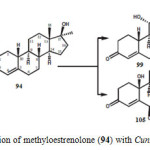 |
Figure 20: Biotransformation of methyloestrenolone (94) with Cunninghamella echinulata. Click here to View figure |
Biotransformation of Anabolic Steroidal Drugs
The term anabolic steroid means any drug or hormonal substance, chemically and pharmacologically related to testosterone (other than estrogens, progestins and corticosteroids), that promotes muscle building. Anabolic–androgenic steroids were first developed in the 1930s to promote the growth of skeletal muscles and to develop male sexual characteristics. They were also used medicinally for the treatment of anemia and osteoporosis. Ethylestrenol (106) is an anabolic steroid, containing a 17-alkyl group with some progestational activity and androgenic effects. It is widely used for the treatment of leucopenia, Sjogren’s syndrome-like disorders and hemophilia B Leyden. Ethylestrenol (106) was fermentated with the plant pathogenic fungus Rhizopus stolonifer afforded 17a-ethyl-3b,17b-dihydroxy-19-norndrost-4-ene (107) and 17a-ethyl-17b-hydroxy-19-norandrost-4-en-3-one (108) as shown in Figure 2120.
Nandrolone (109) is a natural anabolic steroid which occurs in the human body in tiny quantities and it is used for the treatment of autoimmune hemolytic anemia. Nandrolone (109) when incubated with Rhizopus stolonifer, yielded two oxidative metabolites, 19-norandrost-4-en-3,17-dione (110) and 6a,17b-dihydroxy-19-norandrost-1,4-dien-3-one (111) as shown in Figure 2220.
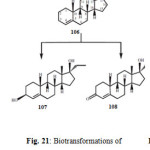 |
Figure 21 Click here to View figure |
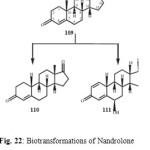 |
Figure 22 Click here to View figure |
Biotransformation of an anabolic synthetic derivative of testosterone, oxandrolone (112), by using suspended-cell cultures of the plant pathogen fungus Rhizopus stolonifer, resulted in the production of three hydroxyl metabolites. These metabolites were identified as 11a-hydroxyoxandrolone (113), 6a-hydroxyoxandrolone (114) and 9a-hydroxyoxandrolone (115) as shown in Figure 23. Metobolite 112 and 114 showed significant b-glucuronidase inhibitory activity21.
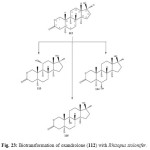 |
Figure 22: Biotransformation of oxandrolone (112) with Rhizopus stolonifer. Click here to View figure |
Conclusion
In conclusion, the current trends in microbial and plant cell-based catalysis of steroids make it possible to predict revolutionary achievements in steroid industry based on “whole-cell based factories” for production of steroid hormones. The advantage in this field is to be focused on “green” solvents and to turn from the two-phase water/organic systems towards more ecologically friendly media. The microbial technology process also provides accesses to regions of the steroidal skeletons, which are difficult to be functionalized by conventional chemical methods. Moreover, laboratory results are characterized as potentially interesting for further industrial applications, but can hardly be used in industrial practice because a cost-efficient industrial process requires a productivity of at least few grams per liter. In order to improve biotransformation processes, problems of low steroid solubility, insufficient substrate availability, and, in some cases, toxicity of substrate/product to microbial cells should be solved.
Acknowledgments
Author was funded by the Fundamental Research Grant Scheme (FRGS) (600-RMI/FRGS 5/3, 12/2012), Ministry of Higher Education, Malaysia.
References
- Zafar S., Yousuf S., Kayani H.A., Saifullah, Khan S., Al-Majid A.M. and Choudhary M.I., Chem. Cent. J. 6: 109-116 (2012).
- Bhatti H.N. and Khera R.A., Steroids. 77: 1267-1290 (2012).
- Donova M.V. and Egorova O.V., Appl. Microbiol. Biotechnol. 94: 1423–1447 (2012).
- Mensah-Nyagan A.G., Meyer L., Schaeffer V., Kibaly C. and Patte-Mensah C., Psychoneuroendocrinology. 34: 169-177 (2009).
- Sedlaczek L., Crit. Rev. Biotechnol. 7: 187-236 (1988).
- Charney W. and Herzong L.H., Microbial transformations of steroids, (Academic Press, New York, 1976) pp. 5–73.
- Hogg J.A., Steroids. 257: 593-616 (1992).
- Mahato M., Phytochemistry. 23: 2131-1254 (1984).
- Liu W.H., Horng W.C. and Tsai M.S., Enzyme Microb. Technol. 18: 184-189 (1996).
- Wang Z., Zhao F., Hao X., Chen D. And Li D., J. Mol. Catal. B 27: 147-153 (2004).
- Kumar R., Dahiya J.S., Singh D. and Nigam P., Bioresour. Technol. 78: 209-211 (2001).
- Choudhary M.I., Erum S., Atif M., Malik R., Khan N.T. and Atta-ur-Rahman. Steroids. 76: 1288-1296 (2011).
- Shah S.A.A., Sultan S., Adnan H.S., Int. J. Pharm. Pharm. Sci. 3: 1-6 (2011).
- Choudhary M.I., Shah S.A.A., Atta-ur-Rahman. Khan S.N. and. Khan M.T.H., Steroids. 75: 956-966 (2010).
- Choudhary M.I., Shah S.A.A., Musharraf S.G., Shaheen F. and Atta-ur-Rahman. Nat. Prod. Res. 17: 215-220 (2003).
- Choudhary M.I., Sultan S., Jalil S., Anjum S., Rahman A.A., Fun H.K. and Atta-ur-Rahman. Chem. Biodivers. 2: 392-400 (2005).
- Shah S.A.A., Sultan S. and Zaimi M. J. Mol. Struct. 1042: 118-122 (2013).
- Baydoun E., Bibi M., Iqbal M.A., Wahab A., Farran D., Smith C., Sattar A.S., Atta-ur Rahman. and Choudhary M.I., Chem. Cent. J. 7: 1-7 (2013).
- Zafar S., Bibi B., Yousuf S. and Choudhary M.I., Steroids. 78: 418-425 (2013).
- Choudhary M.I., Shah S.A.A. and Atta-ur-Rahman. Nat. Prod. Res. 22: 1289-1296 (2008).
- Choudhary M.I., Mohammad M.Y., Musharraf S.G., Parvez M., Al-Aboudic A. and Atta-ur-Rahman. Steroids. 74: 1040-1044 (2009).
- Choudhary M.I., Batool I., Shah S.A.A., Khan S.N. and Atta-ur-Rahman. Nat. Prod. Res. 22: 489-494 (2008).
- Atta-ur-Rahman., Choudhary M.I., Basha F.Z., Abbas G., Khan S.N. and Shah S.A.A., Pure and Appl. Chem. 79: 2263-2267 (2007).
- Choudhary M.I., Yousuf S., Samreen, Shah S.A.A., Ahmed S. and Atta-ur-Rahman. Chem. Pharm. Bull. 54: 927-930 (2006).
- Choudhary M.I., Shah S.A.A., Sami A., Ajaz A., Shaheen F. and Atta-ur-Rahman. Chem. Biodivers. 2: 516-524 (2005)
- Choudhary M.I., Batool I., Shah S.A.A., Nawaz S.A. and Atta-ur-Rahman. Chem. Pharm. Bull. 53: 1455-1459 (2005).
- Atta-ur-Rahman, M.I. Choudhary, S.A.A. Shah, S.N. Khan; US Patent No: 8198041, Date of Patent: Jun. 12 (2012).
- Atta-ur-Rahman, M.I. Choudhary, S.A.A. Shah, S.N. Khan; US Patent No: 8148556, Date of Patent: Apr. 03, (2012).
- Atta-ur-Rahman, M.I. Choudhary, S.A.A. Shah, S.N. Khan; US Patent No: 8148558, Date of Patent: Apr. 03, (2012).
- Atta-ur-Rahman, M.I. Choudhary, S.A.A. Shah, S.N. Khan; US Patent No: 8148557, Date of Patent: Apr. 03, (2012).

This work is licensed under a Creative Commons Attribution 4.0 International License.









