A Validated RP-HPLC Method for Simultaneous Determination of Eberconazole, Mometasone Furoate and Methylparaben
M. Geetha, P. Venkat Rao, Shakil Sait and Sripal Reddy Palvai
Analytical Research and Development, IPDO, Dr. Reddy’s Laboratories Ltd. Bachupally, Hyderabad - 500 072, India.
Corresponding Author E-mail: geetharm@drreddys.com
DOI : http://dx.doi.org/10.13005/ojc/290227
A novel, sensitive and selective isocratic reverse phase high performance liquid chromatographic method was developed and validated for quantitative determination of eberconazole, mometasone furoate and methylparaben in ointment formulations. The chromatographic separation was achieved on Waters Symmetry C-18 (250 x 4.6mm, 5μ) column by using mobile phase containing a mixture of solvent A (0.5g of 1-Hexane sulphonic acid sodium salt and 0.5mL of triethyl amine in 500mL of Milli-Q water, pH 6.0) and B (acetonitrile) in the ratio of 20:80 at flow rate of 1.0 mL/min. Column temperature was maintained at 30°C and detection was carried out at a wavelength of 240 nm. The developed method was validated with respect to specificity, linearity, precision, accuracy and robustness. Forced degradation studies were performed on the placebo and drug product, all degradation products were well separated from eberconazole, mometasone furoate and methylparaben.
KEYWORDS:Eberconazole; Mometasone Furoate; Methylparaben; Ointment
Download this article as:| Copy the following to cite this article: Geetha M, Rao P. V, Sait S, Palvai S. R. A Validated RP-HPLC Method for Simultaneous Determination of Eberconazole, Mometasone Furoate and Methylparaben. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Geetha M, Rao P. V, Sait S, Palvai S. R. A Validated RP-HPLC Method for Simultaneous Determination of Eberconazole, Mometasone Furoate and Methylparaben. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22167 |
Introduction
Eberconazole is a topical, broad-spectrum imidazole derivative, effective in dermatophytoses, candidiasis, and pityriasis treatment. [1] Eberconazole exerts fungicidal effect at high concentrations and fungistatic at low concentration. Eberconazole (nitrate) inhibits the synthesis of ergosterol, an essential component of the cytoplasmic membrane. This leads to an alteration in its structure and function, thereby inhibiting the growth of the fungus. Eberconazole has antifungal as well as potent anti-inflammatory effects. [2, 3] The anti-inflammatory activity attributed to the inhibition of 5-lipoxygenase and to a lesser extent of cyclooxygenase-2. [4]
Mometasone, a synthetic 16 alpha-methyl analogue of beclomethasone, is classified as a ‘potent’ glucocorticoid for dermatological use. [5] Mometasone also referred to as a topical steroid used in addition to emollients for treating inflammatory skin conditions such as eczema or dermatitis. A topical steroid is used when patches of eczema flare up. It is not a cure for your condition, but it will help to relieve the symptoms of a flare-up by reducing inflammation, itching and redness. [6]
Methylparaben is a methyl ester of p-hydroxybenzoic acid. It is a stable, non-volatile compound used as an antimicrobial preservative in foods, drugs and cosmetics for over 50 years. Methylparaben is readily and completely absorbed through the skin and from the gastrointestinal tract. [7] Some studies have reported that all commonly used parabens possess estrogenic activity in several in vitro assays and in animal models in vivo. [8, 9] On long-term exposure at low levels also, parabens retained in human body tissues without hydrolysis by tissue esterases to the common metabolite p-hydroxybenzoic acid. [10] A highly selective and sensitive method is required for the detection of methylparaben.
Literature review reveals that there few HPLC [11-14] and HPTLC [15-16] methods are available for the determination of mometasone furoate in combination with other drugs in different dosage forms. For methylparaben there are several HPLC [17-24] methods available in combined dosage forms. To the best of our knowledge, there is no LC method reported for the simultaneous estimation of eberconazole, mometasone furoate and methylparaben in the gel formulation or determination of eberconazole in single dosage form. Therefore, attempts were made in this study to develop a fast, sensitive, selective and stability-indicating reverse phase liquid chromatography (HPLC) method for the simultaneous determination of eberconazole, mometasone furoate and methylparaben in gel formulation. The proposed method is able to separate eberconazole, mometasone furoate and methylparaben with each other and from its impurities, degradation products and placebo components. The developed LC method was validated with respect to specificity, linearity, precision, accuracy and robustness. Force degradation studies were performed on the placebo and drug product. Developed method separates all degradation products from eberconazole, mometasone furoate and methylparaben and exhibits stability- indicating nature. These studies were performed in accordance with established International Conference on Harmonization (ICH) guidelines.
Experimental
Chemicals and reagents
Ebernet M cream and working standards of eberconazole, mometasone furoate and methylparaben were supplied by Dr. Reddy’s laboratories limited, Hyderabad, India. The HPLC grade acetonitrile and analytical grade Sodium 1-hexane sulphonate, triethyl amine and ortho phosphoric acid were from Merck, Darmstadt, Germany. Water was purified by a Millipore (Bedford, MA, USA) Milli-Q water-purification system and passed through a 0.22 µm membrane filter (Durapore; Millipore, Dublin, Ireland) before use.
Equipment
The waters HPLC system used consists of a Quaternary solvent manager, a sample manager and an UV detector. The output signal was monitored and processed using empower 2 software. Water baths equipped with MV controller (Julabo, Seelbach, Germany) were used for hydrolysis studies.
Chromatographic Conditions
The analytes were separated on Waters Symmetry C-18 (250 x 4.6mm, 5µ) column with mobile phase containing a gradient mixture of solvent A (0.005g of 1-Hexane sulphonic acid sodium salt and 0.5mL of Triethyl amine in 500mL of Milli-Q water, pH 6.0) and solvent B (acetonitrile) in the ratio of 20:80. The mobile phase was filtered through a nylon membrane (pore size 0.45 µm) and degassed with a helium spurge for 10 min, before use. UV detection was performed at wavelength of 240 nm. The sample injection volume was 10 µl.
Diluent: Tetra hydro furan and acetonitrile were used in the ratio of 20:80 v/v as diluent.
Preparation of Standard Solution
Eberconazole nitrate stock solution (a):
Eberconazole nitrate (100 mg) working standard was transferred to 50 mL volumetric flask, dissolved in 30 mL of diluent and volume made up with diluent.
Mometasone furoate stock solution (b):
Mometasone furoate (50 mg) working standard was transferred to 100 mL volumetric flask, dissolved in 70 mL of diluent and volume made up with diluent.
Methyl paraben stock solution (c):
25 mg of Methyl paraben (25 mg) working standard was transferred to 50 mL volumetric flask, dissolved in 30 mL of diluent and volume made up with diluent.
Standard solution:
Eberconazole nitrate stock solution (10 mL) Mometasone furoate stock solution (4 mL) Methyl paraben stock solution (10 mL) were transferred to 50 mL volumetric flask and diluted to volume with diluent.
Preparation of Sample Solution
Sample (2 g-equivalent to 20mg of eberconazole) was transferred into a 50 mL volumetric flask; 10mL of tetra hydro furan was added and sonicated for 15 minutes with occasional shaking. Acetonitrile (20 mL) was added and sonicated for 10 minutes, volume made up to with acetonitrile and mixed well. The solution was centrifuged at 3000rpm for 15 minutes.
Results and Discussion
Method Development and Optimization
The main criteria for development of successful HPLC method for determination of eberconazole, mometasone furoate and methylparaben, the method should be able to separate three analyte peaks and determine the assay value individually from a single analytical method and should be precise, accurate, reproducible, robust, free of interference from blank / placebo / straightforward enough for routine use in quality control laboratory.
The main objective of the chromatographic method was to achieve good resolution between critical closely eluting pair methylparaben and mometasone furoate found symmetrical peak shape of three analytes. To separate all peaks, an elution with 80 % acetonitrile in the mobile phase has been selected. In order to achieve symmetrical peaks of eberconazole, mometasone furoate and methyl paraben and more resolution stationary phases like C18 (different brand), ACE C18 (250 x 4.6, 5µ) column, Xterra RP 18 (100 x 4.6 mm, 5µ), Xterra RP18 (100 x 4.6, 3.5µ) (Fig. 1) were tried but good resolution has achieved with symmetry C18 (250 x 4.6, 5µ) column (Fig. 2). The flow rate of 1.0 mL/min was selected with regards to the backpressure and analysis time as well. Column oven temperature is also studied (30°C and 40°C) and found that 30°C temperature is more appropriate with respect to resolution and peak shape. It was found that use of buffer prepared by adjusting the pH of 6.0 as solvent A and acetonitrile as solvent B in the ratio 20:80 v/v is suitable mobile phase for the good separation. No interfering peaks were observed in blank & placebo, indicating that signal suppression or enhancement by the product matrices was negligible.
Validation of the Method
System Suitability
System suitability parameters were measured so as to verify the system, method and column performance. Standard solution was injected in six replicates, system suitability parameters such as, resolution between three analyte peaks, tailing factor and USP plate count were evaluated, presented in table -1. The results show that method passes the system suitability test.
Table 1: System suitability results
|
Parameter |
Eberconazole |
Mometasone furoate |
Methyl paraben |
|
% RSD Area |
0.1 |
0.1 |
0.1 |
|
% RSD Retention time |
0.6 |
0.5 |
0.7 |
|
USP Tailing |
1.2 |
1.2 |
1.3 |
|
USP Plate count |
13727 |
8448 |
10314 |
|
Resolution |
— |
3.8 |
4.5 |
Specificity
Placebo samples, standard solution, sample solution and blank solution were analyzed in prescribed conditions. The sample and standard chromatograms were identical and there was no interference of placebo.
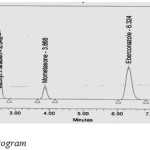 |
Figurew 1: Standard Chromatogram. Click here to View figure |
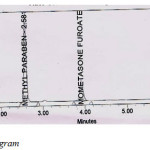 |
Table 1: Sample Chromatogram. Click here to View figure |
 |
Figure 3: Placebo Chromatogram. Click here to View figure |
Degradation studies
To evaluate the specificity of the method further, samples which are degraded under different stress conditions were analysed. The degradation products peaks were well separated from main peaks.
Acidic hydrolysis
The standard solution and 0.1N HCl solution mixed and refluxed for 1 hour at 50oC and then assay of the solution was performed by injecting the solution into system. The resolution between degraded peaks and main peak was good.
Alkaline hydrolysis
The standard solution and 0.1N NaOH solution mixed and refluxed for 15 min. at 25oC and then assay of the drug was performed by injecting the solution into system. The resolution between degraded peaks and main peak was good.
Oxidative stress
The standard solution and 3% H2O2 were refluxed for 2 hours at room temperature and then analysis was performed. Degradation products peaks and drug peak were well separated each other.
Photolytic stress
Exposed to sunlight for about 1.2 million lux hours and UV (shorter and longer wavelengths) for about 200 watt h/square meters. The peaks were well separated from each other.
Thermal stress
The drug was exposed to dry heat at 80oC for 8 hours and analysis performed; the peaks were well separated from each other.
Precision and Intermediated Precision
The assay of formulation was done in six replicates and % RSD was calculated, which was less the 2. To check reproducibility of method Intermediate precision study has been performed in same laboratory with different day on different HPLC system and column. Results are shown in table-3 and Table-4.
Table 2: Results of assay of degradation samples
|
Sample No |
eberconazole |
mometasone furoate |
methylparaben |
|
1 |
97.7 |
97.5 |
99.6 |
|
2 |
99.1 |
99.0 |
100.9 |
|
3 |
97.4 |
97.3 |
99.2 |
|
4 |
99.5 |
99.5 |
101.3 |
|
5 |
98.5 |
98.5 |
100.3 |
|
6 |
97.8 |
97.6 |
99.6 |
|
Average |
98.3 |
98.3 |
100.2 |
|
%RSD |
0.8 |
0.9 |
0.8 |
Table 3: Results of precision
|
Sample No |
eberconazole |
mometasone furoate |
methylparaben |
|
1 |
98.3 |
98.5 |
100.1 |
|
2 |
99.2 |
99.2 |
99.5 |
|
3 |
98.5 |
99.6 |
100.3 |
|
4 |
98.9 |
98.6 |
101.3 |
|
5 |
99.5 |
98.5 |
100.2 |
|
6 |
97.9 |
99.6 |
100.7 |
|
Average |
98.7 |
99.0 |
100.4 |
|
%RSD |
0.6 |
0.5 |
0.6 |
Linearity
Linear calibration plot for the method was obtained over the concentration range from 15% to 150% of standard concentration. The result shows that an excellent correlation existed between the peak area and concentration of the analyte. The correlation coefficient found to be 0.9999, 0.9999 & 0.9997 for eberconazole, mometasone, methylparaben respectively.
Table 4: Results of intermediate precision
|
Sample No |
eberconazole |
mometasone furoate |
methylparaben |
|
1 |
98.3 |
98.5 |
100.1 |
|
2 |
99.2 |
99.2 |
99.5 |
|
3 |
98.5 |
99.6 |
100.3 |
|
4 |
98.9 |
98.6 |
101.3 |
|
5 |
99.5 |
98.5 |
100.2 |
|
6 |
97.9 |
99.6 |
100.7 |
|
Average |
98.7 |
99.0 |
100.4 |
|
%RSD |
0.6 |
0.5 |
0.6 |
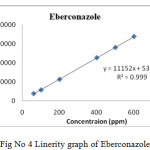 |
Figure 4: Linerity graph of Eberconazole. Click here to View figure |
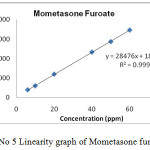 |
Figure 5:Linearity graph of Mometasone furoate. Click here to View figure |
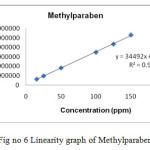 |
Figure 6: Linearity graph of Methylparaben. Click here to View figure |
Accuracy
The standard solution was spiked with predetermined amount of standard at three different concentrations i.e. 50%, 100%, 150% and added amount was evaluated in terms recovery studies. The mean recovery found to be between 98% and 102% which proves method’s accuracy. The results are given in table no 5, 6 and 7
Table 5: Results of Accuracy for Eberconazole
|
Sample No |
Spike level |
mg/mL added |
mg/mL found |
Mean recovery |
|
1 |
50% |
0.1978 |
0.2030 |
102.65 |
|
2 |
50% |
0.1978 |
0.2030 |
|
|
3 |
50% |
0.1978 |
0.2032 |
|
|
1 |
100% |
0.3956 |
0.4037 |
102.04 |
|
2 |
100% |
0.3956 |
0.4047 |
|
|
3 |
100% |
0.3956 |
0.4027 |
|
|
1 |
150% |
0.5934 |
0.5770 |
99.83 |
|
2 |
150% |
0.5934 |
0.5974 |
|
|
3 |
150% |
0.5934 |
0.6028 |
Table 6: Results of Accuracy for Mometasone furoate.
|
Sample No |
Spike level |
mg/mL added |
mg/mL found |
Mean recovery |
|
1 |
50% |
0.02 |
0.0201 |
100.44 |
|
2 |
50% |
0.02 |
0.0201 |
|
|
3 |
50% |
0.02 |
0.0201 |
|
|
1 |
100% |
0.04 |
0.0403 |
100.94 |
|
2 |
100% |
0.04 |
0.0405 |
|
|
3 |
100% |
0.04 |
0.0402 |
|
|
1 |
150% |
0.06 |
0.0590 |
100.52 |
|
2 |
150% |
0.06 |
0.0606 |
|
|
3 |
150% |
0.06 |
0.0611 |
Table 7: Results of Accuracy for Methylparaben
|
Sample No |
Spike level |
mg/mL added |
mg/mL found |
Mean recovery |
|
1 |
50% |
0.0513 |
0.0524 |
102.27 |
|
2 |
50% |
0.0513 |
0.0524 |
|
|
3 |
50% |
0.0513 |
0.0524 |
|
|
1 |
100% |
0.1025 |
0.1036 |
101.10 |
|
2 |
100% |
0.1025 |
0.1039 |
|
|
3 |
100% |
0.1025 |
0.1034 |
|
|
1 |
150% |
0.1538 |
0.1478 |
98.73 |
|
2 |
150% |
0.1538 |
0.1531 |
|
|
3 |
150% |
0.1538 |
0.1545 |
Robustness
To evaluate the robustness of the method, experimental conditions were deliberately altered and the system suitability tests were performed. To study the effect of flow rate on the performance flow was changed by 0.2 units from 0.8 to 1.2 mL/min. The effect of the column temperature on performance was studied at 25° and 35° C instead of 30° C. The effect of the percent organic strength was studied by varying acetonitrile by −10% and +10%. While other mobile phase components were held constant. In all the deliberate varied chromatographic conditions (flow rate, column temperature and composition of organic solvent), the resolution between critical pairs, i.e. imp-D and imp-E was greater than 2, tailing factor less than 2, theoretical plate count more than 5000 and %RSD of peak area & retention time proves the robustness of the method.
Stability in Solution and in the Mobile Phase
The solution stability of standard and test solution ware carried out in tightly capped volumetric flasks at room temperature for 24 hours. Contents of eberconazole, methylparaben and Mometasone furoate were determined after 12 and 24 hours. No significant changes were observed in the content of Eberconazole, Mometasone furoate and methylparaben.
Conclusions
The isocratic HPLC method developed for determination of eberconazole, mometasone furoate and methylparaben in pharmaceutical dosage forms is specific, sensitive, precise, accurate, linear and robust. The described method is suitable for stability studies, routine analysis, and quality control of creams and ointments or other pharmaceutical preparations containing eberconazole, mometasone furoate and methylparaben either alone or in combination.
Acknowledgement
We wish to express our sincere thanks to the Management of Dr. Reddys Laboratories, Hyderabad, India for their support and encouragement. Cooperation from colleagues and Analytical Research & Development of Dr.Reddy’s Laboratories Ltd. is appreciated.
References
- Repiso Montero T, López S, Rodríguez C, del Rio R, Badell A, Gratacós MR; Int. J. Dermatol., 45, 600-604 (2006).
- LathaSubramanyaMoodahadu-Bangera, Jacinta Martis, Rajan Mittal, BinnyKrishnakutty, Naveen Kumar, Shantala Bellary, Sunoj Varughese, Parinitha K. Rao; Indian Journal of Dermatology, Venereology and Leprology, 78, 217-222 (2012).
- Laboratorios Salvat, S.A (Innovators). Product information. 2001.
- Dr. Reddys Laboratories. DRL (Innovators). Product information. 2007.
- Prakash A, Benfield P; Drugs, 55, 145-63 (1998).
- www.patient.co.uk/medicine/Mometasone-(topical).htm.
- Soni MG, Taylor SL, Greenberg NA, Burdock GA;Food Chem Toxicol. 40, 1335-73 (2002).
- Routledge EJ, Parker J, Odum J, Ashby J, Sumpter JP; Toxicol ApplPharmacol., 153, 12-19 (1998).
- Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong W, Shi L, Perkins R, Sheehan DM; Toxicol Sci., 54, 138-53 (2000).
- Manuela M. Mincea, Ioana R. Lupşa, Dan F. Cinghita, Ciprian V. Radovan, Ioan Talpos and Vasile Ostafe. J. Serb. Chem. Soc., 74, 669–676 (2009).
- X W Teng, K Foe, K F Brown, D J Cutler, N M Davies; J Pharm Biomedi Anal., 26, 313-19 (2001).
- Shaikh S, Muneera MS, Thusleem OA, Tahir M, and Kondaguli AV; Journal of Chromatographic Science, 47(2), 178-183 (2009).
- Katari Srinivasa rao, Vinayk Gorule, Venkata Reddiah Chand Venkata Krishna A; J Anal. Bioanal. Techniques doi:10.4172/2155- 9872.1000153 (2012)
- Kabeer Ahmed Shaikh and Ashish Tanaji Patil; Journal of Trace Analysis in Food and Drugs, 1, 14-21 (2013)
- Amol A. Kulkarni, Rabindra K. Nanda, Meenal N. Ranjane, Poonam N. Ranjane; Der Pharma Chemica, 2, 25-30 (2010)
- Hiral N. Dave, Ashlesha G. Makwana, Chaitanya Bhatt, and Killambi Pundari kakshudu; International Journal of Applied Science and Engineering, 9, 177-185 (2011).
- G. A. Shabir; Indian J Pharm Sci., 72, 421–425 (2010).
- BahruddinSaad, Md. Fazlul Bari, Muhammad IdirisSaleh, Kamarudzaman Ahmad, Mohd. KhairuddinMohd. Talib; J. Chromatogr. A, 1073, 393–397(2005)
- R. Hájková, P. Solich, M. Pospısilova, J. Sicha; Analytica Chimica Acta, 467, 91–96 (2002)
- Manuela M Mincea, Ioana R Lupsa, Dan F Cinghita, Ciprian V. Radovan, Ioan Talpos and Vasile Ostafe; J. Serb. Chem. Soc., 2009; 74, 669–676 (2009).
- Rajesh M Kamble, Santosh G. Singh and Shrawan Singh; E-Journal of Chemistry, 8, 340-346 (2011)
- A. Abdollahpour, M. Forouhi, M. Shamsipur and Y. Yamini; J. Iran. Chem. Soc.,7, 516-520 (2010).
- P. Solich, R. Hajkova, M. Pospisilova, J. Sicha; Chromatographia, 56, S181-S184 (2002)
- S. Gopalakrishnan, T.A. Chitra, A. Aruna and A.Chenthilnathan; Der Pharma Chemica, 4, 1003-1015 (2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.









