Mixed- Ligand Phosphine and Arsine Complexes of Ruthenium (III) Ligated by Heterocyclic Thioamide
R. N. Pandey*, A. K. Nag and D. K. Sharma
P.G. Centre of Chemistry (M.U.) College of Commerce, Patna - 800 020, India.
Article Received on :
Article Accepted on :
Article Published : 21 Oct 2016
Mixed ligands bis-chelates of general formula [RuX(E??????????3)(IPT5T)2] (X= Cl, Br, NCS or NO3; E= P/As) have been prepared and investigated. The ligand 1-phenyl tetrazoline -5-thione acts as bidentate (N,S) supported by IR, and 1HNMRspectral data. The distorted octahedral structure of all Ru(III) compounds are assigned on the basis of elemental, magnetic and other physico-chemical data.
KEYWORDS:Ru(III); bis-chelates; Mixed- ligands
Download this article as:| Copy the following to cite this article: Pandey R. N, Nag A. K, Sharma D. K. Mixed- Ligand Phosphine and Arsine Complexes of Ruthenium (III) Ligated by Heterocyclic Thioamide. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Pandey R. N, Nag A. K, Sharma D. K. Mixed- Ligand Phosphine and Arsine Complexes of Ruthenium (III) Ligated by Heterocyclic Thioamide. Available from: http://www.orientjchem.org/?p=22774 |
Introduction
Organometallic complexes of ruthenium are important class of compounds having great catalytic 1-4, medicinal5-7 and biological properties8-10. The present paper is devoted to the synthesis and characterization of new ruthenium(III) complexes with
1-phenyl tetrazoline-5-thione (I).
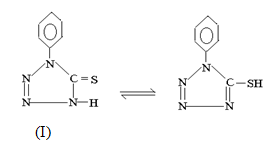
Experimental
All the chemicals used were of Analar or Cp grade. Solvents were dried prior to use. 1-phenyl tetrazoline-5-thione was prepared by the method of Liber et al11. The starting complexes [RuX3(EΦ3)3]12-14 (X= Cl, Br; E= P/As) were prepared according to the literature procedures.
Preparation of [RuX(EΦ3)(IPT5T)2] (X= Cl, Br, NCS or NO3; E= P/As)
All reactions were carried out under anhydrous conditions and complexes were prepared using a general method:
Ethanolic solution of ligand and benzene solution of precursor’s complexes were mixed in molar ratio 2:1(L:M) and refluxed for 5 hours. The resulting dark coloured solution was concentrated to ~ 5cm3 and some small quantity of ether was added to it. The precipitated brown coloured solids were filtered, washed with ether and dried in vacuo.
For the preparation of thiocyanate and nitrate complexes, ethanolic solution of NH4SCN/LiNO3 was added to benzene solution of [RuX(EΦ3)3] and refluxed with solution of ligand using desired molar ratio. The complexes were obtained by the concentration of refluxed solution to ~5cm3 and addition of small quantity of ether.
[Ru(PΦ3)(1PT5T)2Cl]:
Sl.No. 1: calculated (%) for RuC32H25N8S2PCl: C,51.02; H, 3.32;N,14.88;Ru,13.42;
Found (%): C,51.15; H, 3.38;N,14.90;Ru,13.42;
[Ru(PΦ3)(1PT5T)2Br]:
Sl.No. 2: calculated (%) for RuC32H25N8S2PBr: C,48.18; H, 3.13;N,14.05;Ru,12.67;
Found (%): C,48.35; H, 3.10;N,14.12;Ru,12.87;
[Ru(AsΦ3)(1PT5T)2Cl]:
Sl.No. 3: calculated (%) for RuC32H25N8S2AsCl: C,48.21; H, 3.13;N,14.06;Ru,12.68;
Found (%): C,48.11; H,3.25;N,14.21;Ru,12.72;
[Ru(AsΦ3)(1PT5T)2Br]:
Sl.No. 4: calculated (%) for RuC32H25N8S2AsBr: C, 45.66; H, 2.97; N, 13.32;Ru,12.01;
Found (%): C, 45.69; H, 3.01; N,13.42;Ru,12.32;
[Ru(PΦ3)(1PT5T)2NCS]:
Sl.No. 5: calculated (%) for RuC33H25N9S3P: C,51.09; H, 3.22;N,16.25;Ru,13.03;
Found (%): C,51.13; H,3.01;N,16.22;Ru,13.13;
[Ru(PΦ3)(1PT5T)2(NO3)]:
Sl.No. 6: calculated (%) for RuC32H25N9S2O3P: C,49.29; H, 3.29;N,16.17;Ru,12.96;
Found (%): C,49.39; H,3.30;N,16.27;Ru,13.01;
The analysis of carbon, hydrogen and nitrogen were performed at CDRI Lucknow, India. The IR spectra of ligand and complexes were recorded on a Perkin-Elmer Model-577 spectrophotometer in the range of 4000-200 cm-1as KBr pillets. The magnetic measurements were made on a Gouy balance and the diamagnetic corrections for the ligand molecule were applied. The UV and Visible spectra of the ligand and complexes were recorded on a Beckmann and Carl Zeiss(Jenna) spectrophotometer. The molar conductance of complexes(10-3M) were measured in DMF (10-3M) using Wiss-Werkstatter Weitheim obb type LBR conductivity meter. 1H NMR spectra of ligand and Ru(III) complexes were recorded with 90 MHZ NMR spectrophotometer using TMS as internal indicator. The complexes were dissolved in CDCl3 for recording their 1HNMR spectra in the range of 0-10 ppm. The number of protons were obtained with the help of internal calibrator. EPR spectra of the powdered samples were recorded on a Bruker E-112 Varian model instrument in X-band frequencies at room temperature.
Results and Discussions
The analytical data of the complexes correspond to the composition [RuX(EΦ3)(1PT5T)2] (X=Cl, Br, NCS, NO3; E=P/As; 1PT5T= deprotonated 1-phenyl tetrazoline-5-thione of tautomeric form. The molar conductance values indicate their non-electrolytic nature and magnetic moment value of the complexes (table-1) fall i9n the range of 1.90-2.01 BM corresponding to a single unpaired electron in low spin 4d5 configuration consistent with reported value reported in previous literature15.
The room temperature EPR spectra of powered sample were recorded at X-band frequencies. All the complexes showed a single isotropic resonance with a “g” value in the range 2.10-2.30 ranges indicating high symmetry around ruthenium(II) ion16. Such isotropic lines are probably due to the results of either intermolecular spin exchange which can broaden the lines or due to occupancy of unpaired electron in degenerate orbital17. The values of “g” is also in agreement with low spin symmetry of the ligand field similar to “g” value reported for octahedral
Ru (III) complexes18-20.
Electronic spectra of complexes showed two to three bands in the 250-670 nm region. The band in the 535-485 nm region have been assigned to the 2T2g→ 2A2g transitions is in conformity with assignments made for similar ruthenium (III) complexes21-23. Other bands in the 345-355 nm region have been assigned to charge transfer transition and the other two bands at 675 and 470 nm are spin-forbidden transitions24. In general the electronic spectra of all complexes are characteristic of an octahedral environment around Ru (III) ions.
IR Spectra:
A close observation of infra red spectrum of ligand and ruthenium (III) complexes indicate simultaneous Ru-S and Ru-N bonding in all complexes. The υ N-H at 3145cm-1 of ligand 1-phenyltetrazoline-5-thione disappears from the spectra of complexes indicating deprotonation of N-H proton. Further evidence in support of this comes from systematic shift25-27 of thiomide bands of the ligand on complexation. The formation of simultaneous Ru-S and Ru-N bond blue shift thiomide band I (20-35cm-1) , band III
(25-30 cm-1) and band IV(30-40 cm-1) of ligand due to increase in CN bond order and decrease in CS bond order28-29. These observations are further supported by the presence of new non-ligand bond at 420 cm-1 and 350 cm-1 assigned to υRu-N and υRu-S
modes respectively.
The non-ligand bands at Ca. 1505,1350 and 1000 cm-1 correspond to υ4, υ1 and υ2 vibration of coordinated nitrate group in [Ru(NO3)(PΦ3)(1PT5T)2] are in agreement with previous literature30.Separation between the two bands υ4 and υ1(ca. 155 cm-1) indicate the monodentate nature of nitrate group Ca. 1800 cm-1 are not observed in the spectrum of the present complex. A very strong band of medium intensity at 2085cm-1, 760 cm-1 and 480 cm-1 confirms the presence of N-bonded isothiocynato group and assigned to υNCS , υC=S and δCNS modes 31.
1H NMR Spectra:
All complexes display broad multiplet in the region δ7.45-7.75 ppm due to phenyl protons in complexes. The broad nature of peak may be due to large quadruple resonance broadening effect of tetrazoline nitrogen atom32. The resonances due to imino proton in the ligand observed at δ1.25 ppm is absent in the spectra of the complexes suggesting formation of Ru-N bond and deprotonation of N-H group on complexation. The aromatic protons of PΦ3 ligand resonated as a broad multiplet in the region δ7.32-7.15 ppm in complexes.
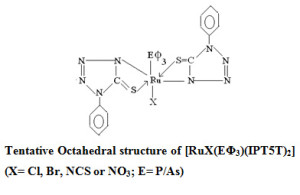
Table-1.
Physico-chemical data of Ru(III) complexes.
|
Compound |
μeH(BM) |
λ max.(nm) |
Thiomide Bands I II III IV |
|
Ligand (1PT5TH) |
– |
305,265 |
1520 1290 980 740
|
|
1.[Ru(PΦ3)(1PT5T)2Cl]
|
2.01 |
675, 485, 345 |
1500 1300 1000 800 |
|
2. [Ru(PΦ3)(1PT5T)2Br]
|
1.90 |
670, 470, 350 |
1515 1295 970 795 |
|
3. [Ru(AsΦ3)(1PT5T)2Cl]
|
2.00 |
660, 355 |
1520 1310 965 760 |
|
4. [Ru(AsΦ3)(1PT5T)2Br]
|
2.01 |
665, 350 |
1510 1310 975 745 |
|
5. [Ru(PΦ3)(1PTST)2(NCS)]
|
1.90 |
675, 470, 355 |
1515 1315 980 740 |
|
6. [Ru(PΦ3)(1PT5T)2NO3]
|
1.90 |
605,535,345 |
1525 1315 985 745 |
REFERENCES:
- M.A. Bennett, M.I. Burce and T.W.Matheson; Compresensive Organometallic chemistry, vol 4, Edited by I. Wiekinson, F.G.A. stone, E.W. Abel(Pergamon, Oxford) P. 796(1982).
- P.Mulle and C. Fruit, chem.. Rev. 103,2905(2003).
- S.Yamada, Coord.chem. Rev. 191, 537(1999).
- E.c. Niederhoffer, j.H. Timmons and A.E. Martell, Chem. Rev. 84, 137(1984).
- K.Karidi, A.Garoufic, A.Tsipis, N.Hadjiliadis, H. Den Dulk and J. Reedijik, J. Chem. Soc. Dalton. Trans, 1176(20050.
- P.I.Anderberg, M.M. harding, I.J. Luck and P.Turner, Inorg. Chem.. 41, 1365(2001).
- B.R.Cameron, M.C. darkes, I.R. Bird, R.t. Skerli, Z.L. santucci and S.P. Fricker, Ingor.Chem. 42, 4102(2003).
- Pattrringg and J.a. grim, cancerRes. 27, 1278(1967).
- F.a. farach, E.J. Bianz, S.C. Sadlin and N.Brackman; J. Med. Chem.. 17,172(1974).
- K.P.Balasubramanian, s. Manivannan and V. Chinnusamy, J. Ultra. Chem. Vol.4(1), 15(2008).
- E.Liber and J. ramchandran, can J. Chem. 37, 101(1959).
- J.Chatt, G.j. Leigh, D.M.P. Mingos and r.J. Paske; J. Chem.. Soc. A 2636(1968).
- R.K. Poddar, I.P.Khullar and U.Agarwala, Nucl. Chem. Lett. 10, 221(1974).
- K.Natrajan, R.K. Poddar and U.Agarwala, J. Inorg. Nucl. Chem. 39, 431(1977).
- N.Prasanna, S.Srinivasan, G. Rajagopal and P.R. Athappan, Indian J. Chem. 40A,426(2001).
- T.D.Thangadurai and K.Natarajan, Indian J. chem.. 40A, 573(20001).
- P.Viswanathanmurthi and K.Natarajan, Indian J. Chem. 38A, 797(1999).
- T.D. Thangadurai and K. Natarajan, Indian J. Chem. 41A, 741(2002).
- R.Ramesh, N.Dharmraj, R.Karvembu and K.Natarajan, Indian j. Chem. 39A,1079(2000).
- K.P. Balasubramannian, R. Karvembu, V. Chinnusamy and K.Natrajan, Indian J.Chem. 44A, 2450(2005).
- ABP Lever, “Ingorganic electronic Spectroscopy”, Elsevier, N. Y. Second Edn.(1984).
- C.J. Bullhausen, “ Intrduction to Ligand Field Theory”, Mc Graw-Hill, N.Y., (1962)P. 276.
- L.R. Ramirez, T.A. Stephenson and E.S. Switkes, J. Chem. Soc. A, 1770(1973).
- K.P. Balasubramanian, K. Parameshwari, V. Chinnusamy, R. Prabhakaran and K.Natarajan, Spectrochim. Acta. 65A, 678(2006).
- R.N. Pandey, R. Bala and A.K. Sinha, Oriental J. Chem. Vol. 27(no.1), 293(2011).
- B. Singh, r. singh, R V Choudhary and K.P. thakur, Indian J. Chem. 11, 174(1973).
- R. N. Pandey, A. Anand, R.K. Singh and A.Kumar, Asian J. Chem. Vol 22(No.7),5601(2010).
- U. Agarwala and B.Singh, Indian J. Chem. 7, 726(1969).
- R.N. Pandey and Rajnish Kumar singh, Oriental J. Chem. 25, 599(2009).
- G. Mathew and M.L.H.K. Nair, Asian J. Chem. Vol 16, (N0.3-4), 1875(2004).
- R.N. Pandey and R.N. Sharma, J. Ultra Chem. Vol 7(3), 391(2011).
- R.N. Pandey and Sheo Shankar Kumar, J. Ultra Chem. Vol 7(2), 271(2011).
Table-1.
Physico-chemical data of Ru(III) complexes.
|
Compound |
μeH(BM) |
λ max.(nm) |
Thiomide Bands I II III IV |
|
Ligand (1PT5TH) |
– |
305,265 |
1520 1290 980 740
|
|
1.[Ru(PΦ3)(1PT5T)2Cl]
|
2.01 |
675, 485, 345 |
1500 1300 1000 800 |
|
2. [Ru(PΦ3)(1PT5T)2Br]
|
1.90 |
670, 470, 350 |
1515 1295 970 795 |
|
3. [Ru(AsΦ3)(1PT5T)2Cl]
|
2.00 |
660, 355 |
1520 1310 965 760 |
|
4. [Ru(AsΦ3)(1PT5T)2Br]
|
2.01 |
665, 350 |
1510 1310 975 745 |
|
5. [Ru(PΦ3)(1PTST)2(NCS)]
|
1.90 |
675, 470, 355 |
1515 1315 980 740 |
|
6. [Ru(PΦ3)(1PT5T)2NO3]
|
1.90 |
605,535,345 |
1525 1315 985 745 |

This work is licensed under a Creative Commons Attribution 4.0 International License.









