Synthesis and Characterization of Mercury(Ii) Complexes Containing Mixed Ligands of Mono or Diphosphines and Saccharinate
Shihab A.O. Al-Dury and Subhi A. Al-Jibori*
Department of Chemistry, College of Science, University of Tikrit, Tikrit (Iraq).
Tetrahedral mercury(II) complexes of the types [HgCl(sac)(PPh3)2], [HgCl(sac)(diphos)], [Hg(sac)2(PPh3)2] or [Hg(sac)2(diphos)] and octahedral complexes of the type [Hg(sac)2(dppe)2] or [Hg(sac)2(dppp)2] {diphos = Ph2P(CH2)nPPh2; n=1, dppm; n=2, dppe; n=3, dppp; n=4,dppb} were prepared and characterized by molar conductance, elemental analysis, infrared spectra, 1H and 31P-{1H} nmr data.
KEYWORDS:Mercury; Saccharinate; Diphosphine
Download this article as:| Copy the following to cite this article: Al-Dury S. A. O, Al-Jibori S. A. Synthesis and Characterization of Mercury(Ii) Complexes Containing Mixed Ligands of Mono or Diphosphines and Saccharinate. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Al-Dury S. A. O, Al-Jibori S. A. Synthesis and Characterization of Mercury(Ii) Complexes Containing Mixed Ligands of Mono or Diphosphines and Saccharinate. Available from: http://www.orientjchem.org/?p=23408 |
Introduction
Saccharin{o-sulfobenzimide; 1,2-benzothiazol-3(2H)-one-1,1-dioxide; Hsac}, most widely used as an artificial sweetening agent Interaction of saccharin. with different biologically relevant cations attracted great interest due to the suspected carcinogenicity of this compound [1-3] which was definitively ruled out in 2001 [3-4]. Saccharin ligand has three potential donor sites, it is not expected to use all three towards the same metal because of geometrical constraints. It is therefore likely to acts as a monodentate or a bidentate . Metal Complexes of this ligand with transition and non transition metals have been studied extensively these have been comprehensively revied [5] and a large number of papers published every year in this field. Saccharinate interacts with some heavy non.-transition metal cations such as Cd(II) and Hg(II) to yield of [Cd(sac)2(H2O)2].2H2O. [6] and [Hg(sac)2] [7] respectively. Mixed ligands Hg(II) complexes containing saccharinato and nitrogen donor ligands have been reported [8-14]. However mixed ligand complexes of Hg(II) with saccharin and mono or diphosphine seems to be unexplored although such mixed ligand complexeshave been reported for some other transition metals [15-17] We expect that mixed ligands complexes of tertiary phosphines and saccharin to be an important class of complexes and exhibit synergic effect attributed to the mixed ligands.
In the present paper we report the synthesis and characterization of some mercury(II) complexes containing mixed ligands, tertiary, mono or diphosphines and saccharin.
Experimental
General
The experimental techniques were the same as those used in our recent paper from this laboratory [18].
Starting materials
The compounds HgCl2, Hg(OAc)2, dppm, dppe, dppp, dppb, PPh3 and Nasac were commercial products and were used as supplied. The compounds [HgCl(sac)] and [Hg(sac)2] were prepared according to literature methods [19,20].
Preparation of complexes
[HgCl(Sac)(dppm)]2(1)
A solution of dppm (0.07g, 1.9 mmol.) in warm EtOH (7ml) was added to a suspension of [Hg(sac)Cl] (0.08g, 1.9 mmol.) in hot EtOH (10ml). The mixture was stirred at room temperature for 1h. The pale white solid thus formed was filtered off washed with EtOH, dried under vacuum (yield 67%).The following complexes were prepared and isolated by a similar method; (2),(3) and (4).
[Hg(sac)2(dppm)] (5)
A solution of dppm (0.05g, 0.1 mmol.) in warm EtOH (7ml) was added to a suspension of [Hg(sac)2] (0.08g, 0.1 mmol.) in hot EtOH (7ml). The resulting clear solution was filtered off and evaporated. The pale white solid thus formed was filtered off washed with EtOH, dried under vacuum and recrystalized from DMSO, (yield 95%)
[Hg(sac)2(dppe)] (6)
A solution of dppe (0.07g, 0.18 mmol.) in warm EtOH (7ml) was added to a suspension of [Hg(sac)2] (0.1g, 0.18 mmol.) in hot EtOH (10ml). The mixture was stirred at room temperature for 1h. The pale white solid thus formed was filtered off washed with EtOH, dried under vacuum (yield 66%). The following complexes were prepared and isolated by a similar method; (7) and (8).
[Hg(sac)2(dppe)2] (9)
A solution of dppe (0.079g, 0.17 mmol.) in warm EtOH (10ml) was added to a suspension of [Hg(sac)2] (0.059g, 0.088 mmol.) in hot EtOH (10ml). The resulting clear solution was filtered off and evaporated to near dryness. n-Hexane (10ml) was added, The pale white solid thus formed was filtered off washed with EtOH, dried under vacuum (yield 89%). The following complexes were prepared and isolated by a similar method; (10), (11) and (12).
Table 1: Color, Yield, Elemental analysis and conductivity of complexes (1) – (12)
|
(Ohm-1.cm2.mol-1) L |
|
Found(cal.)% |
Yield% |
Color |
Complexes |
Seq |
||||
|
CH2Cl2 |
DMSO |
CHCl3 |
CH3OH |
N |
H |
C |
||||
|
– |
5.9 |
– |
– |
2.4 (2.1) |
3.7 (3.9) |
43.0 (42.7) |
67 |
White |
[HgCl(sac)(dppm)]2 |
1 |
|
2.8 |
– |
– |
– |
1.4 (1.5) |
5.6 (5.7) |
53.8 (53.6) |
60 |
White |
[HgCl(sac)(dppe)] |
2 |
|
3.1 |
– |
– |
– |
2.8 (2.9) |
3.4 (3.4) |
48.0 (48.1) |
80 |
White |
[HgCl(sac)(dppp)] |
3 |
|
1.9 |
– |
– |
– |
3.2 (3.1) |
4.5 (4.5) |
48.2 (48.5) |
95 |
White |
[HgCl(sac)(dppb)] |
4 |
|
– |
2.3 |
– |
– |
1.6 (1.6) |
4.6 (4.4) |
51.1 (50.8) |
95 |
White |
[Hg(sac)2(dppm)] |
5 |
|
– |
4.3 |
– |
– |
1.7 (1.6) |
4.4 (4.3) |
51.0 (50.9) |
66 |
White |
[Hg(sac)2(dppe)] |
6 |
|
– |
– |
– |
0.9 |
2.6 (2.5) |
5.3 (5.3) |
54.9 (54.6) |
69 |
White |
[Hg(sac)2(dppp)] |
7 |
|
– |
|
2.1 |
– |
2.9 (2.6) |
4.6 (4.6) |
53.4 (53.1) |
76 |
White |
[Hg(sac)2 (dppb)] |
8 |
|
– |
– |
– |
1.4 |
2.0 (2.2) |
4.8 (4.8) |
57.0 (57.1) |
89 |
White |
[Hg(sac)2(dppe)2] |
9 |
|
– |
– |
– |
2.4 |
2.2 (1.9) |
5.0 (4.8) |
60.2 (60.1) |
89 |
White |
[Hg(sac)2(dppp)2] |
10 |
|
– |
– |
3.2 |
– |
3.0 (2.7) |
4.6 (4.4) |
52.7 (52.6) |
95 |
White |
[Hg(sac)Cl(PPh3)2] |
11 |
|
– |
– |
– |
2.1 |
2.3 (2.3) |
4.5 (4.7) |
58.1 (58.2) |
89 |
White |
[Hg(sac)2 (PPh3)2] |
12 |
( Table 2 : I.R. spectraa data cm-1 of the ligands and complexes (1) – (12
|
u(P-C) |
u(P-Hg) |
(SO2) |
(CNS) |
u (CN) |
u (CC) |
u (CO) |
u (C-H) |
Complexes |
Seq. |
|||
|
uas |
us |
uas |
us |
Alp. |
Ar. |
|||||||
|
1275s 1257v |
1145v1126s |
966s |
1336m |
1450m |
1585m |
1643vs |
3099w 3072w |
[Nasac] |
|
|||
|
1286vs 1251vs |
1153vs |
968s |
1338m |
1456m |
1583m |
1697vs 1635vs |
3078w |
[HgCl(sac)] |
|
|||
|
1257s |
1153m |
960s |
1342s |
1413m |
1577m |
1645m |
[[Hg(sac)2] |
|
||||
|
513m |
335vs |
1253s |
1147vs |
945m |
1330m |
1430s |
1575m |
1620s |
2924w 2864w |
3051w |
[HgCl(sac)(dppm)]2 |
1 |
|
515m |
350vs |
1294vs 1247m |
1155vs |
947s |
1340m |
1433m |
1581m |
1634s |
2906w |
3057w |
[HgCl(sac)(dppe)] |
2 |
|
519m |
335vs |
1286s 1250s |
1149vs |
951s |
1340w |
1430s |
1570m |
1641s |
2935w |
3055w |
[HgCl(sac)(dppp)] |
3 |
|
519m |
330vs |
1290vs 1246s |
1151vs |
957s |
1330w |
1438m |
1575m |
1686s 1645s |
2935w |
3055w |
[HgCl(sac)(dppb)] |
4 |
|
532m |
335vs |
1290vs 1240s |
1151vs |
950s |
1334m |
1444m |
1568s |
1645vs |
2926w 2866w |
3059w |
[Hg(sac)2(dppm)] |
5 |
|
522m |
338vs |
1290vs 1244s |
1159vs |
954vs |
1330m |
1442m |
1581m 1564m |
1662vs |
2964w 2916w |
3059w |
[Hg(sac)2(dppe)] |
6 |
|
524m |
335vs |
1288s 1247s |
1149vs |
951s |
1330m |
1442m |
1568s |
1643s |
2914w |
3059w |
[Hg(sac)2(dppp)] |
7 |
|
532m |
335vs |
1288s 1257s |
1151vs |
953s |
1336m |
1425m |
1572s |
1645s |
2926w 2868w |
3059w |
[Hg(sac)2(dppb)] |
8 |
|
522m |
338vs |
1286vs 1247s |
1147vs |
951vs |
1325m |
1431m |
1577m |
1639s |
2962w 2906w |
3057w |
[Hg(sac)2(dppe)2] |
9 |
|
524m |
335vs |
1278s 1253s |
1147vs |
949s |
1330m |
1433m |
1577s |
1635vs |
2922w |
3057w |
[Hg(sac)2(dppp)2] |
10 |
|
511s |
332vs |
1292vs 1240s |
1153vs |
951s |
1330w |
1433s |
1585w |
1661s |
– |
3056W |
[Hg(sac)Cl(PPh3)2] |
11 |
|
513s |
352vs |
1290s 1252s |
1151vs |
953s |
1330w |
1431m |
1570m |
1649m |
3056W |
[Hg(sac)2(PPh3)2] |
12 |
|
Table 3: The 1H and 31P-{1H} n.m.r. dataa of the prepared complexes.
|
Complexes |
Seq. |
dP |
dCH2 |
2J(199Hg-31P) |
dPh.P. |
Solvent |
|
[HgCl(sac) (dppm)]2 |
1 |
23.59 |
3.3 |
5166 |
7.37-7.88 |
DMSO |
|
[HgCl(sac) (dppe)] |
2 |
31.12 |
3.27 |
– |
7.38-7.84 |
DMSO |
|
[Hg(sac)2(dppe)] |
6 |
45.00 |
– |
– |
– |
DMSO |
|
[Hg(sac)2(dppp)] |
7 |
38.56 |
2.13-3.1 |
5519 |
7.27-7.38 |
CD3OD |
|
[Hg(sac)2(dppe)2] |
9 |
21.96 |
3.12 |
2195 |
7.16-7.75 |
CD3OD |
|
[Hg(sac)2(dppp)2] |
10 |
15.0 |
1.378-2.994 |
2031 |
7.355-7.67 |
CD3OD |
Results and Discussion
Synthesis of complexes
It was reported previously [19] that reaction of HgCl2 with sodium saccharinate in aqueous medium gives [[HgCl(sac)]. Single crystal X-ray diffraction showed that this complex is linear with N-Hg-Cl bond angle 177.6 (3)o. We have previously reported [18] that two coordinate linear mercury(II) complexes may permit for coordination number to be extended to four or may be six. Treatment of the linear mercury(II) complex [HgCl(sac)] with one mole proportion of the diphosphines Ph2P(CH)nPPh2 (n=1-4) or two mole proportion of PPh3 gave tetrahedral complexes of the type [HgCl(sac)(µ-Ph2PCH2PPh2)]2(1) or the [HgCl(sac){Ph2P(CH)nPPh2}] (n=2,3 or 4) (2), (3), (4), or the [HgCl(sac){PPh3)2](11).
Treatment of the linear mercury(II) complex [Hg(sac)2] [20] with one mole proportion of the diphosphines Ph2P(CH)nPPh2 (n=1,2,3 or 4) or two moles proportion of PPh3 gave tetrahedral complexes of the type [Hg(sac)2(diphos)] (5), (6), (7), (8), or [Hg(sac)2{PPh3)2](12). However treatment with two moles of the diphosphine gave octahedral complexes of the type [Hg(sac)2(dppe)2](9) or [Hg(sac)2(dppp)2](10).
Characterization of complexes
The prepared complexes were characterized by elemental analysis, i.r. spectra, conductivity measurements and some of then by 31P-{1H} and 1H nmr spectra and their data are listed in tables 1-3. The molar conductivity of the complexes in DMF, CH3OH, CHCl3, DMSO or CH2Cl2 is low enough to suggest that they one non-electrolytes [21].
Nuclear magnetic resonance
The 31P-{1H} and 1H nmr data of some of the prepared complexes are given in Table 3. The 31P-{1H} nmr spectrum of [HgCl(Sac)(dppm)]2(1) showed a singlet at dP=23.59ppm with 2J(199Hg-31P)=5166Hz. The positive dP value indicates that dppm behaves as a bidentate bridging [15,22-24]. This has been supported by the 1H – {31P} nmr spectrum which showed a singlet at dH=3.3ppm assigned for the methylene protons of the bridging dppm [25]. The large 2J(199Hg-31P) which is 5166Hz indicated a tetrahedral geometry around mercury[26-28]. The 31P-{1H} nmr spectra of the other tetrahedral complexes (2), (6) and (7) each showed a singlet at dP=31.12, 45.00 and 38.56ppm respectively. Complex(7) showed 2J(199Hg-31P)=5519Hz which suggest four coordinate tetrahedral arrangement around mercury. The 31P-{1H} nmr spectra for [Hg(sac)2(dppe)2](9) and [Hg(sac)2(dppp)2](10) showed a singlet each at dP=21.96 and 15ppm respectively. The 2J(199Hg-31P) values for these two complexes were low 2195 and 2031Hz respectively which suggest a six coordinate environment around mercury [26-28 ].
On the basis of the above nmr data and other identification data given in Tables 1 and 2, the structures shown in figure 1 have beensuggested
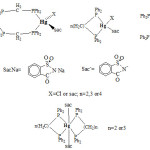 |
Figure 1: Suggested structures for the prepared mercury(II) complexes
|
Infrared spectra
Selected infrared spectroscopic data of the prepared complexes (1)–(12) are listed in Table2. All complexes display a sharp strong bands between (1620-1697cm-1) attributed to the u(C=O) of the saccharin ligand coordinated as monodentate though the nitrogen atom [29,30]. Two strong bonds at around (1240-1294) and (1147-1153cm-1) are characteristic for the uas SO2 and us SO2 modes of sac, respectively. Whereas the bands at ca. (1325-1342) and (949-968cm-1) are assigned to the symmetric and asymmetric stretching of the CNS moiety in the sac ion. Additional bands between 332-352cm-1 assigned to u(P-C).The u(C-H) aliphatic appeared at ca. 2906-2935cm-1 while the u(C-H) aromatic appeared at ca. 3051-3059cm-1.
Conclusion
In summery reaction of the linear mercury(II) complex [HgCl(sac)] with mono or diphosphine resulted in the formation of tetrahedral complexes of the type [HgCl(sac)(diphos)]. Reaction of [Hg(sac)2] with one mole equivalent of diphos. gave tetrahedral complexes of the type [Hg(sac)2(diphos)] while reaction with two mole equivalent gave octahedral complexes of the type [Hg(sac)2(diphos)2].
Acknowledgment
We would like to thank the nmr department Institute for Anorganische Chemic, Martin- luther- University, Halle, Germany for measuring the nmr spectra.
References
- N. Suzuki and H. Suzuki; Cancer Res., 1995, 55, 4253.
- J. Zurlo and R.A. Squire; J.Natl-Cancer Inst., 1998, 90, 2.
- M. R. Weihrauch, V. Diehl and H.Bohlen; Mediz. Klin., 2001, 96, 670.
- Chem. Brit., 2001, 37, 18.
- E.G. Baran and V.T. Yilmaz; Coord.Chem. Rev., 2006, 250, 1980.
- S.Z. Haider, K.M.A. Malik, S. Das, M.B. Hursthouse; Acta Crystallogr., 1984, C40 1147.
- B. Kamenar, G. Jovanovski, D. Grdenic; Cryst. Struct. Commun., 11, 1982, 263.
- A. Hergold-Brundic, B. Kamenar, and G. Jovanovski; Acta Crystallogr., 1989, C45, 556.
- O. Grupce, G. Jovanovski, B. Kaitner and P. Naumov; Croat. Chem. Acta.,1999, 72, 465.
- Y. Topcu, O. Andac, V.T. Yilmaz and W.T.A. Harrison; Cryst. Res. Technol., 2002, 37, 509.
- V.T. Yilmaz, S. Hamamci, and C. Thoene; Cryst. Res. Technol., 2002, 37, 1143.
- V.T. Yilmaz, S. Caglar, and W.T.A. Harrison; Z. Anorg. Allg. Chem., 2004, 630, 1512.
- Z. S. Seddigi, A. Banu and G. M. G. Hossain, The Arabian J. for Science and Engineering,. 2007, 32, 181.
- R.M.K. Deng, K.B. Dillon, A.E. Goeta, M. Mapolelo and H.J. Shepherd; Inorg. Chim. Acta., 2009, 362, 5109.
- W. Henderson, B. K. Nicholson, and L. J. McCaffrey., Inorg. Chim. Acta., 1999, 285, 145.
- L. R. Falvello, J. Gomes,I. Pascual, M. Tomas, E. P. Urriolabeitia and A. G. Schultz; Inorg. Chem., 2001, 40, 4455.
- W. Henderson, B. K. Nicholson, and D.C. Chung; Acta Crystallographica, Section E. Structure reported Online., 2002, E58, m432.
- A.S.M. AL-Janabi, B.H. Abdullah and S.A. Al-Jibori; Orient. J. Chem., 2009, 25(2), 277.
- G. Jovanovski, B. Kamenar, G. Ferguson, and B. Kaitner., Acta Crystallogr., 1988, C44, 616.
- B. Kamenar, G. Jovanovski and D. Grdenic; Cryst. Struct Commun., 1982, 11, 263.
- W.J. Geary; Corrd. Chem. Rev., (1971), 7, 81.
- C.T. Hunt and A.L. Balch; Inorg. Chem., 1981, 20, 2267.
- L. J. Al-Hayaly, B. H. Abdullah, A.A.N. Al-Dulaimi and S.A. Al-Jibori; Orient. J. Chem., 2008, 24(2), 38.
- S. A. Al-Jibori, A. S. S. Al-Zaubi, M. Y. Mahammed and T. A. K. Al-Allaf ; Trans. Met. Chem., 2007, 32, 398.
- E.C. Aleya, S.A. Dias, R.G. Goel, W.O. Ogini, P. Pilom and D.W. Meek; Inorg. Chem., 1978, 17, 1697.
- P.G. Pringle, D. Phill Thesis, Leeds University, U.K. (1983).
- P.G. Pringle and B.L. Shaw; J. Chem. Soc. Dalton Trans., 1983, 5, 861.
- M.D. Lumsden, K. E.ichele, R.E. Wasylishen, T.S. Cameron and J.F.Britten; J. Am. Chem. Soc., 1994, 116, 11129.
- P. Naumov, and G. Jovanovski; J. Mol. Struct., 2001, 263/264, 335.
- P. Naumov, and G. Jovanovski; Curr. Org. Chem., 2001, 5, 1059.

This work is licensed under a Creative Commons Attribution 4.0 International License.









