Synthesis of Some Thiazino-pyrazoles as Potential Fungicides
Priyanka Kedia,Vandana Singh and Daroga Singh*
*Synthetical Organic Research Lab., Department of Chemistry, T.D.P.G. College, Jaunpur - 222 002 (India).
4-Arylmethylene-2,4-dihydro-2-arylthioanilido-5-methyl-3H-pyrazol-3-ones(2) were prepared by the condensation of 2,4-dihydro-2-arylthioanilido-5-methyl- 3H- pyrazol - 3- ones (1) with aromatic aldehydes in the presence of glacial acetic acid. The synthesized compounds (2) reacted with phenyl thiourea in presence of alcoholic KOH at reflux temperature to give 1-arylthioanilido-3-methyl-4-aryl-6-phenylimino -4,7- dihydro-1, 3- thiazino [5,4-d] pyrazoles (3). The synthesised compounds (3) have been screened for their fungicidal activities against fungi A. fumigatus and C. albicans at different concentrations.
KEYWORDS:Pyrazolone; thiazono-pyrazoles; Antifungal activity; Thiazono-pyrazoles
Download this article as:| Copy the following to cite this article: Kedia P, Singh V, Singh D. Synthesis of Some Thiazino-pyrazoles as Potential Fungicides. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Kedia P, Singh V, Singh D. Synthesis of Some Thiazino-pyrazoles as Potential Fungicides. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=24016 |
Introduction
Pyrazole derivatives possess diverse chemical reactivity and broad spectrum of biological activities . A number of substituted pyrazoles are used as fungicides, insecticide, pesticides, herbicides in the field of agriculture (1,2). They are used as colouration of paints, varnishes, lacquers in the field of industry (3,4) . These derivatives are used as anti-oxidant(5) for rubber , linseed oil and as disperse dyes (6). They possess wide range of pharmacological activities as hypoglycemic(7), antimicrobial(8,9), anticonvulsive(10), anti-inflammatory(11), antihistaminic(12), anti coagulant(13), and anti-rheumatic(14) agents etc. Some thiazino-pyrazole derivatives have been synthesized and screened their antimicrobial activity(15-17). In continuation of our study an attempt is to synthesize some new derivatives of thiazino-pyrazole and screened their fungicidal activity against both the test fungi A. fumigatus and C. albicans at different concentration viz, 1000,100 & 10 ppm.
2,4-dihydro-2-arylthioanilido-5-methyl-3H-pyrazol – 3 – ones (1) were synthesised by the reaction of ethylacetoacetate and aryl thiosemicarbazide. The compound (1) was condensed with aromatic aldehyde in presence of glacial acetic acid to give 4-arylmethylene – 2, 4-dihydro- 2- arylthioanilido -5-methyl-3H-pyrazol-3-ones(2).The compound(2) was refluxed with phenyl thiourea in presence of alcoholic KOH for 3-4 hrs to give 1-arylthioanilido-3-methyl-4-aryl-6-phenylimino-4,7-dihydro-1,3-thiazino[5,4-d]pyrazoles(3)(Scheme-1).
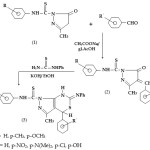 |
Scheme 1 Click here to View scheme |
Experimental
Melting points were taken in an open capillary tube and are uncorrected. The IR spectra were recorded in KBr disc on Perkin-Elmer-720 spectrophotometer. The 1H NMR spectra were recorded in CDCl3 /DMSO-d6 on Varian A-60D spectrophotometer. The chemical shifts are recorded in d ppm down field from TMS, which are used as an internal standard.
2,4-Dihydro-2-arylthioanilido-5-methyl-3H-pyrazol-3-ones(1):
These pyrazolones were prepared by known method(18,19).
4-Arylmethylene – 2,4-dihydro- 2-arylthioanilido-5-methyl-3H-pyrazol – 3-ones(2):
A mixture of compound (1)(0.01mol), aromatic aldehyde (0.01 mol) and sodium acetate (0.011 mol) was refluxed in glacial acetic acid for 2h. The reaction mixture was cooled and poured in cold water to give solid materials. The resulting solid was recrystallised from ethanol.
1-Arylthioanilido-3-methyl-4-aryl-6-phenylimino-4,7-dihydro-1,3-thiazino[5,4-d]pyrazoles(3):
These were prepared by know method(20).A mixture of compound (2) (0.01 mol), potassium hydroxide (0.02 mol) and phenyl thiourea (0.01 mol) was refluxed for 3-4 h in ethanol. After evaporating the solvent , it was then acidified with dilute hydrochloric acid to give solid materials. The resulting solid was recrystallised from ethanol. The physical and spectral data of all synthesised compounds (3a-o) are given in the table-1.
Table 1
|
Comp. No. |
R |
R| |
M.P. (0c) |
Yield (%) |
I.R. (KBr disc) cm–1, nmax |
PMR (CDCl3) d (ppm) |
|
3a |
H |
H |
158 |
65 |
3510(NH), 3390(=NPh), 1530(C=N),1230(C=S) |
2.0(3H,s,CH3), 4.2(1H,s,S-CH),
6.9-7.6(15H,m,ArH), 8.6(2H,s,NH) |
|
3b |
H |
p-NO2 |
178 |
61 |
3515(NH), 3395(=NPh), 1535(C=N),1235(C=S) |
2.1(3H,s,CH3), 4.0(1H,s,S-CH),
6.8-7.8(14H,m,ArH), 8.8(2H,s,NH) |
|
3c |
H |
p-N(Me)2 |
182 |
72 |
3518(NH), 3398(=NPh), 1538(C=N),1234(C=S) |
2.3(3H,s,CH3),3.0(6H,s,NMe2),
4.1(1H,s,S-CH), 6.9-7.8(14H,m,ArH) , 8.9(2H,s,NH) |
|
3d |
H |
p-Cl |
195 |
68 |
3505(NH), 3390(=NPh), 1532(C=N),1230(C=S) |
2.0(3H,s,CH3), 4.3(1H,s,S-CH),
6.8-7.5(14H,m,ArH), 8.8(2H,s,NH) |
|
3e |
H |
p-OH |
214 |
70 |
3580(OH), 3520(NH), 3390(=NPh), 1535(C=N),1238(C=S) |
2.0(3H,s,CH3), 4.1(1H,s,S-CH),
6.5(1H,s,OH), 7.0-7.5(14H,m,ArH), 8.9(2H,s,NH) |
|
3f |
p–CH3 |
H |
157 |
62 |
3525(NH), 3395(=NPh), 1540(C=N),1236(C=S) |
2.1(3H,s,CH3), 3.4(3H,s,ArCH3),
4.4(1H,s,S-CH), 7.1-7.9(14H,m,ArH), 8.7(2H,s,NH) |
|
3g |
p–CH3 |
p-NO2 |
179 |
64 |
3514(NH), 3398(=NPh), 1545(C=N),1238(C=S) |
1.9(3H,s,CH3), 3.2(3H,s,ArCH3),
4.3(1H,s,S-CH), 7.2-8.0(13H,m,ArH), 8.9(2H,s,NH) |
|
3h |
p–CH3 |
p-N(Me)2 |
193 |
69 |
3515(NH), 3395(=NPh), 1535(C=N),1235(C=S) |
1.8(3H,s,CH3), 3.0(6H,s,NMe2),
3.5(3H,s,ArCH3), 4.6(1H,s,S-CH), 7.1-7.6(13H,m,ArH), 8.6(2H,s,NH) |
|
3i |
p–CH3 |
p-Cl |
202 |
70 |
3520(NH), 3390(=NPh), 1542(C=N),1236(C=S) |
2.1(3H,s,CH3), 3.3(3H,s,ArCH3),
4.1(1H,s,S-CH), 6.9-7.9(13H,m,ArH), 8.7(2H,s,NH) |
|
3j |
p–CH3 |
p-OH |
218 |
72 |
3585(OH), 3508(NH), 3398(=NPh), 1538(C=N),1240(C=S) |
2.2(3H,s,CH3), 3.4(3H,s,ArCH3),
4.2(1H,s,S-CH), 6.4(1H,s,OH), 7.2-7.7(13H,m,ArH), 8.8(2H,s,NH) |
|
3k |
p–OCH3 |
H |
154 |
68 |
3505(NH), 3390(=NPh), 1545(C=N),1245(C=S) |
1.9(3H,s,CH3), 3.7(3H,s,OCH3),
4.3(1H,s,S-CH), 7.1-7.9(14H,m,ArH), 8.6(2H,s,NH) |
|
3l |
p–OCH3 |
p-NO2 |
165 |
65 |
3512(NH), 3380(=NPh), 1548(C=N),1250(C=S) |
1.8(3H,s,CH3), 3.8(3H,s,OCH3),
4.4(1H,s,S-CH), 6.9-7.8(13H,m,ArH), 8.7(2H,s,NH) |
|
3m |
p–OCH3 |
p-N(Me)2 |
186 |
68 |
3528(NH), 3384(=NPh), 1530(C=N),1255(C=S) |
2.2(3H,s,CH3), 3.1(6H,s,NMe2), 3.7(3H,s,OCH3), 4.1(1H,s,S-CH),
7.2-8.0(13H,m,ArH), 8.8(2H,s,NH) |
|
3n |
p–OCH3 |
p-Cl |
219 |
73 |
3505(NH), 3380(=NPh), 1540(C=N),1258(C=S) |
2.1(3H,s,CH3), 3.6(3H,s,OCH3),
4.5(1H,s,S-CH), 6.9-8.0(13H,m,ArH), 8.6(2H,s,NH) |
|
3o |
p–OCH3 |
p-OH |
207 |
71 |
3590(OH), 3500(NH), 3382(=NPh), 1545(C=N),1260(C=S) |
2.0(3H,s,CH3), 3.5(3H,s,OCH3),
4.6(1H,s,S-CH), 6.4(1H,s,OH), 7.2-8.0(13H,m,ArH), 8.8(2H,s,NH) |
Antifungal Activity
All the thiazino-pyrazoles (3a-o) were screened for their antifungal activity against Aspergillus fumigatus and Candida albicans by using food poisoning of solidified agar techniques at various concentration viz. 1000, 100 and 10 ppm by the standard method(21).
The inhibition of the fungus growth was determined as the difference in growth between test and control plates. The percentage inhibition in the colony of the test fungus was expressed as –
Percentage inhibition (%) = (C–T) × 100/C
where C – diameter of fungus colony (mm) in control plates.
T – diameter of fungus colony (mm) in treated plates.
The antifungal activity in terms of percentage inhibition shown by thiazino-pyrazoles (3a-o) has been listed in the Table 2.
Table 2
|
Comp. No. |
Substituents |
Average % inhibition after 96 hours |
||||||
|
R |
R| |
Organism A. fumigatus concentration (ppm) used |
Organism C. albicans concentration (ppm) used |
|||||
|
1000 |
100 |
10 |
1000 |
100 |
10 |
|||
|
3a |
H |
H |
56.4 |
47.2 |
36.2 |
55.2 |
46.0 |
35.2 |
|
3b |
H |
p-NO2 |
59.2 |
49.4 |
39.4 |
58.0 |
48.2 |
38.2 |
|
3c |
H |
p-N(Me)2 |
60.4 |
50.2 |
40.8 |
59.2 |
49.2 |
39.6 |
|
3d |
H |
p-Cl |
62.6 |
52.2 |
42.8 |
61.4 |
50.0 |
40.8 |
|
3e |
H |
p-OH |
55.2 |
44.4 |
35.2 |
54.0 |
43.2 |
34.4 |
|
3f |
p–CH3 |
H |
55.0 |
46.0 |
35.0 |
54.8 |
45.8 |
34.2 |
|
3g |
p–CH3 |
p-NO2 |
58.0 |
48.2 |
38.2 |
57.6 |
47.2 |
37.2 |
|
3h |
p–CH3 |
p-N(Me)2 |
59.2 |
49.4 |
39.4 |
58.0 |
48.2 |
38.2 |
|
3i |
p–CH3 |
p-Cl |
61.6 |
51.8 |
41.2 |
60.4 |
49.4 |
39.0 |
|
3j |
p–CH3 |
p-OH |
54.2 |
42.6 |
34.0 |
53.0 |
41.2 |
33.2 |
|
3k |
p–OCH3 |
H |
54.0 |
45.8 |
34.2 |
53.8 |
44.4 |
33.4 |
|
3l |
p–OCH3 |
p-NO2 |
56.8 |
47.0 |
37.4 |
55.6 |
46.2 |
36.0 |
|
3m |
p–OCH3 |
p-N(Me)2 |
57.4 |
48.8 |
38.2 |
56.2 |
47.4 |
37.8 |
|
3n |
p–OCH3 |
p-Cl |
60.4 |
50.2 |
39.8 |
59.2 |
48.0 |
38.2 |
|
3o |
p–OCH3 |
p-OH |
53.2 |
40.2 |
33.2 |
52.0 |
40.2 |
31.8 |
| Bavistin |
99.4 |
95.0 |
90.0 |
99.2 |
95.0 |
90.0 |
||
Result and Discussion
It was observed from the antifungal screening data that all the compounds (3a-o) show antifungal activity against both the test fungi A. fumigatus and C. albicans. It was also observed that the fungicidal activity of all synthesised compounds decreased upon dilution,so that all the compounds show greater fungitoxicity as the concentration increased . Structure activity relationship have demon- strated that the electron withdrawing group (deactivating group)
increase the fungicidal activity and electron donating group (activating group) decrease the fungicidal activity(22). The compounds having p-Cl group (3d) inhance the antifungal activity than the p-N(Me)2 group (3c). Since p-Cl group is electron withdrawing group and p-N(Me)2 group is electron donating group. Similarly we compare the compounds having p-NO2 group (3b) inhance the fungicidal activity than the p-OH group (3e) . Over all result in my experiment from antifungal screening data that the p-Cl group inhance the fungicidal activity than p-NO2 & p-N(Me)2.
Acknowledgement
The authors are very grateful to Dr. U. P. Singh, Principal and the Head , Deptt. of chemistry , T.D.P.G.College, Jaunpur (U. P.) for providing necessary facilities.
References
- Kidwai M. and Mohan R. ; Journal of Korean Chemical Society , 48, No. 2(2004).
- Tripathi P. and Pandey S.; Asian Journal of Chemistry , 20 , No.1, 808(2008).
- Ricoh Co.Ltd., Japan Kokai, Tokkyo, Koho, 8069, 148(1980);cf C.A. ,93, 248206 F(1980).
- Ramanathan V. ; U.S., 3, 954, 398(1978); cf; C.A., 85, 79675K (1976).
- Howland, U.S. Pat., 2,458,780,1949.
- Decb A. , Yassin F., Ouf N. and Shehta W. ; Chemistry of Heterocyclic compounds , 46,212 (2010).
- Cottineau Bertrand, Toto Bertrand, Marot Christophe, Pipaud Aline, Chenault Jacques; Bioorganic & Medicinal Chemistry Letters, 12 ,2105(2002).
- Deshmukh M.B., Deshmukh S.A., Jagtap S.S., Suryavanshi A.W. , Jadhav S,D. and Anbhule P.V. ; J. Indian Chem. Soc. , 86, 613 (2009).
- Bandock Samir, Fadalya Walid and Metwallya M.A. ; European Journal of Medicinal Chemistry, 45,3692(2010).
- Ozdermir Z., Kandilei H.B. , Gumusel B., Calis U. and Bilgin A.A.; Eur. J. Med. Chem., 42,373(2007).
- Saky Subas M. et al., Bioorganic & Medicinal chemistry letters, 18, 1042(2008).
- Sridevi C.H., Balaji K. , Naidu A. and Subhakaran R. ; E J Chem. 7, 234(2010).
- Viehlyaev, Grandberg and Kost, Formakal; I. Toksikol.,25,27(1962).
- Garg H.G. and Kaur N. ; J. Med. Chem., 15,554(1972).
- Yadav A.K., Singh Vandana & Singh Daroga, Asian Journal of Chemistry, 19,No.5, 3511(2007).
- Yadav A.K. , Singh Sobha, Singh Vandana and Singh Daroga , Acta Ciencia Indica, Vol.XXXIIC, No.1, 013, (2007).
- Singh Vandana , Kedia Priyanka and Singh Daroga , J. Chemtracks, 11, 157, (2009).
- Burrus H.O. and Powell G.; J. Am. Chem. Soc., 67, 1468 (1945).
- Singh Daroga and Singh D. ; J. Indian Chem. Soc., 68, 165 (1991).
- Tiwari Nirupama, Dwivedi Bandana, Ali Roab and Nizamuddin; J. Indian Chem. Soc., 67, 521 (1990).
- Horsfall J.G., Bot. Rev., 11, 357(1945).
- Neuvonen Kari, Neuvonen Helmi and Fulop Ferenc; Bioorganic & Medicinal Chemistry Lettres, 16, 3495(2006).

This work is licensed under a Creative Commons Attribution 4.0 International License.









