Synthesis, Characterization and Biological Activity of Novel Heterocyclic Compounds Containing Acylated Pyrazoline
Sandip U Agare1 , Mahesh P More1
, Mahesh P More1 , Sanjay P Tajane2
, Sanjay P Tajane2 and Tanuja V Kadre1*
and Tanuja V Kadre1*
1Department of Chemistry, Dr. A.P.J. Abdul Kalam University, Indore, Madhya Pradesh, India.
2Department of Chemistry, GITAM Deemed to be University, Vishakhapatnam, Andhra Pradesh, India.
Corresponding Author E-mail: tanujakadre45@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400223
Article Received on : 19 Dec 2023
Article Accepted on :
Article Published : 11 Mar 2024
Reviewed by: Dr. Farzaneh Mohamadpour
Second Review by: Dr. Panshina Svetlana
Final Approval by: Dr. Ayssar Nahle
A set of novel acylated pyrazoline compounds 3(a-i) was prepared and structural elucidation was confirmed by using spectroscopic techniques such as 1H-NMR, FT-IR, and mass spectroscopy. Progress of the reaction was monitored by TLC. The synthesized set of acylated pyrazoline compounds 3(a-i) was evaluated for antimicrobial screening which reveals that these compounds have interesting properties. The discs diffusion method was utilized to perform in-vitro antimicrobial activity of pyrazoline derivatives 3(a-i) against Escherichia coli (MCC-2412), Staphylococcus aureus (MCC-2408), B. subtilis (MCC-2010), P. aeruginosa (MCC-2080), Saccharomyces cerevisiae (MCC-1033), and Candida albicans (MCC-1439).Some of the acylated molecules such as 3c, 3d, 3g and 3e emerged as excellent designs represents comparable or higher antimicrobial activities concerning standard drug candidates.
KEYWORDS:Antifungal; Antibacterial; Acylated Pyrazoline; Antimicrobial; Pyrazoline
Download this article as:| Copy the following to cite this article: Agare S. U, More M. P, Tajane S. P, Kadre T. V. Synthesis, Characterization and Biological Activity of Novel Heterocyclic Compounds Containing Acylated Pyrazoline. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Agare S. U, More M. P, Tajane S. P, Kadre T. V. Synthesis, Characterization and Biological Activity of Novel Heterocyclic Compounds Containing Acylated Pyrazoline. Orient J Chem 2024;40(2). Available from: https://bit.ly/4a94USD |
Introduction
In recent years fungal infections have been a significant health issue affecting individuals globally 1. The advent of antibiotics for combating fungus and other microorganisms has led to pharmaceutical–resistant infections. The use of pharmacological agents including Fluconazole, Azathioprine, Methotrexate, Voriconazole, Cyclosporine, Intraconazole, Posaconazole, Micafungin, Flucytosine, Caspofungin, Anidulafungin, and others are efficacious in combating, fungal infections. However, it has been observed that the administration of these pharmaceuticals in conjunction with other medications has resulted in the occurrence of adverse effects 2, 3. However, the presence of fluorine in the heterocyclic compound has shown a significant influence on its biological activity. Several previous researchers have reported the synthesis of pyrazoline by reacting fluorinated chalcones and hydrazine hydrated or its analogous possess considerable biological antimicrobial, anticancer, antifungal, and anti-inflammatory activities 4,5. The Structure fluconazole one of the most widely used antifungal drugs, bearing two triazoles and another part containing a di-fluoro aryl ring is an active pharmacophore site has been envisaged, and modifying this geometry would have an impact on pharmacological activity 6-8. The presence of fluorine substituent in the aromatic ring had enhanced antifungal efficacy against numerous microbial strains 9-13. Currently worldwide demands safe and potent antifungal antimicrobials drugs combat several common as well as life-threatening infections with no adverse impact. This extensive diversity in pharmacological belonging to pyrazoline molecules has encouraged our research team to venture into this research area. This research work reports the novel fluorine-induced acylated pyrazoline candidates and their antibacterial and antifungal biological activities. This work continues our search for novel biologically active compounds containing potent acylated pyrazoline candidates which will be helpful for future novel drug designing.
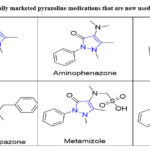 |
Table 1: Commercially marketed pyrazoline medications that are now used in clinical practice. |
Materials and methods
The Barnstead Electrothermal 9100 melting point apparatus was used to determine the m.p. (uncorrected). The deuterated chloroform (CDCl3) served as the solvent for the proton NMR spectra performed on Bruker Avance-400 MHz spectrometer. IR data were recorded by the FTIR-BRUKER instrument. The HRMS was captured using a Bruker IMPACT HD instrument. To monitor and detect the chemicals, purity was checked by TLC. The spots on the sheets were seen under a UV light at 254 nm. There was no need for additional purification because all of the analytical-grade chemicals and reagents were used.
Synthesis of fluorinated chalcones 2(a–i)
In ethanol (25 mL), a mixture of 4-fluoro-3-methylacetophenone (1) (10 mmol) and cyano- and chloro-substituted benzaldehydes (a-i) (10 mmol) was taken in ethanol solvent. To the above solution was added 2N aqueous Sodium hydroxide (15 mL) dropwise at 0±5 °C. After completion of the addition, the ice bath was removed and the reaction mass was stirred at ambient temperature for 2h. The pH of the reaction medium was adjusted to 5, by aq. 2N HCl solution. Solid precipitated was filtered, washed with water, and dried on a rotatory evaporator to yield the dry solid. The corresponding chalcones 2(a–i) were purified through the process of recrystallization using ethanol. TLC was used to monitor the reaction progress 14.
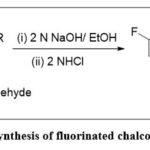 |
Scheme 1: Synthesis of fluorinated chalcones 2(a–i). |
Synthesis of acylated pyrazolines 3(a–i):
A solution containing a combination of chalcones 2(a–i) (10 mmol) with the required amount of hydrazine hydrate (20 mmol) in glacial acetic acid was subjected to reflux for an extended period. The reaction mixture underwent a cooling process and was subsequently transferred into crushed ice. The resulting residue was then separated using filtration, followed by a washing and drying procedure, resulting in the formation of candidates 3(a–i). The advancement of the reaction was checked using TLC 15.
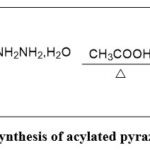 |
Scheme 2: Synthesis of acylated pyrazolines 3(a–i). |
Spectral description of 3 (a-i)
Candidate (3a)
White solid, yield: (214 mg, 66.67 %), m. p: 188±1 °C; FT-IR (cm-1): 3063(CO–CH3), 2924 (-CH3), 2229 (nitrile CN), 1719 (keto group C=O), 1630 (Pyrazoline C=N), 1502/1400 (Ar-ring C=C), 1362 (C-F), 1012 (N-N); 1H NMR (400 MHz, CDCl3):d ppm 2.331 (s, 3H), 2.414 (s, 3H), 3.112-3.156 (dd, 1H), 3.774-3.848 (dd, 1H), 5.622-5.621 (dd, 1H), 7.073-7.117 (m, 1H), 7.473-7.638 (m, 6H). HRMS (m/z): 321.5542. Anal. calcd for C19H16FN3O: C, 71.01; H, 13.02; F, 5.91; N, 13.08; O, 4.98.
Candidate (3b)
White solid, yield: (249 mg, 71.55 %), m. p: 183±1 °C; IR (cm-1): 3065(CO–CH3), 2926 (-CH3), 2229 (CN), 1725 (keto group C=O), 1605 ((Pyrazoline C=N), 1501/1411 (Ar-ring C=C), 1363 (C-F), 1037 (N-N); 1H NMR (400 MHz, CDCl3): d ppm 2.331 (S, 3H), 2.414 (s, 3H), 3.112-3.156 (dd, 1H), 3.774-3.848 (dd, 1H), 5.622-5.621 (dd, 1H), 6.917-6.988 (m, 2H), 6.991-7.100 (m, 2H), 7.288-7.342 (m, 1H), 7.5328-7.638 (m, 2H). HRMS (m/z): 322.9871. Anal. calcd for C19H16FN3O: C, 71.01; H, 13.02; F, 5.91; N, 13.08; O, 4.98.
Candidate (3c)
White solid, yield: (256 mg, 73.56 %), m. p: 178±1 °C; IR (cm-1): 3038(CO–CH3), 2920 (-CH3), 2227 (CN), 1652 (keto group C=O), 1605 (Pyrazoline C=N), 1502/1409 (Ar-ring C=C), 1331 (C-F), 1016 (N-N); 1H NMR (400 MHz, CDCl3): d 2.356 (s, 3H), 2.458 (s, 3H,), 3.102-3.158 (dd, 1H), 3.775-3.849 (dd, 1H,), 5.616-5.658 (dd, 1H), 7.069-7.114 (dd, 1H), 7.299-7.381 (m, 2H), 7.550-7.615 (m, 1H), 7.646-7.666 (m, 3H). HRMS (m/z): 322.9874. Anal. calcd for C19H16FN3O: C, 71.01; H, 5.02; F, 5.91; N, 13.08; O, 4.98.
Candidate (3d)
White solid, yield: (256 mg, 70.01 %), m. p: 199±1 °C; IR (cm-1): 3067(CO–CH3), 2925 (-CH3), 1739 (keto group C=O), 1605 (Pyrazoline C=N), 1502/1402 (Ar-ring C=C), 1323 (C-F), 1010 (N-N), 818 (C-Cl); 1H NMR (400 MHz, CDCl3): d ppm 2.343 (s, 3H), 2.520 (s, 3H), 3.056-3.10 (dd, 1H), 3.841-3.915 (dd, 1H), 5.930-5.970 (dd, 1H), 6.989-7.008 (m, 1H), 7.050-7.094 (m,1H), 7.157-7.211 (m, 1H), 7.300 (m,1H), 7.422-7.548 (m, 1H), 7.599-7.616 (m, 1H). HRMS (m/z): 363.1474. Anal. calcd for C18H15Cl2FN3O: C, 59.19; H, 4.14; Cl, 19.41; F, 5.20; N, 7.67; O, 4.38.
Candidate (3e)
White solid, yield: (272 mg, 74.52 %), m. p: 188±1 °C; IR (cm-1): 3067(CO–CH3), 2925 (-CH3), 1739 (keto group C=O), 1605 (Pyrazoline C=N), 1502/1402 (Ar-ring C=C), 1323 (C-F), 1010 (N-N), 818 (C-Cl); 1H NMR (400 MHz, CDCl3): d ppm 2.300 (s, 3H), 2.449 (s, 3H), 3.050 (dd, 1H), 3.810 (q, 1H), 5.858-5.899 (t, 1H), 7.015-7.051 (m, 1H), 7.073-7.095 (m, 1H), 7.212-7.232 (m, 1H), 7.450 (m, 1H), 7.545-7.559 (m, 1H), 7.601-7.618 (m, 1H). HRMS (m/z): 364.2769. Anal. calcd for C18H15Cl2FN3O: C, 59.19; H, 4.14; Cl, 19.41; F, 5.20; N, 7.67; O, 4.38.
Candidate (3f)
White solid, yield: (263 mg, 73.42 %), m. p: 183±1°C; IR (cm-1): 3088(CO–CH3), 2925 (-CH3), 1740 (keto group C=O), 1586 (Pyrazoline C=N), 1502/1438 (Ar-ring C=C), 1362 (C-F), 1008 (N-N), 817 (C-Cl); 1H NMR (400 MHz, CDCl3): d ppm 2.345 (s, 3H), 2.523 (s, 3H), 2935 (dd, 1H), 3.820-3.894 (q, 1H), 5.866-5.907 (dd, 1H), 7.039-7.199 (dd, 2H), 7.220-7.262 (dd, 1H), 7.295-7.373 (dd, 1H), 7.547-7.624 (dd, 2H). HRMS (m/z): 365.2291. Anal. calcd for C18H15Cl2FN3O: C, 59.19; H, 4.14; Cl, 19.41; F, 5.20; N, 7.67; O, 4.38.
Candidate (3g)
White solid, yield: (298 mg, 81.64 %), m. p: 194±1 °C; IR (cm-1): 3052(CO–CH3), 2929 (-CH3), 1741 (keto group C=O), 1581 (Pyrazoline C=N), 1501/1432 (Ar-ring C=C), 1320 (C-F), 1017 (N-N), 821 (C-Cl); 1H NMR (400 MHz, CDCl3): d ppm 2.360 (s, 3H,) 2.392 (s, 3H), 3.275-3.40 (q, 1H), 3.665-3.742 (q, 1H,), 6.225-6.278 (q, 1H), 7.069-7.091 (q, 1H), 7.113-7.192 (dd, 1H), 7.277-7.295 (dd, 1H), 7.542-7.556 (dd, 1H), 7.563-7.570 (dd, 1H), 7.575-7.661 (dd, 1H). HRMS (m/z): 365.0028. Anal. calcd for C18H15Cl2FN3O: C, 59.19; H, 4.14; Cl, 19.41; F, 5.20; N, 7.67; O, 4.38.
Candidate (3h)
White solid, yield: (255 mg, 69.86 %), m. p: 182±1 °C; IR (cm-1): 3066(CO–CH3), 2925 (-CH3), 1587 (keto group C=O), 1587 (Pyrazoline C=N), 1502/1402 (Ar-ring C=C), 1321 (C-F), 1013 (N-N), 819 (C-Cl); 1H NMR (400 MHz, CDCl3): d ppm 2.359 (s, 3H), 2.469 (s, 3H), 3.091-3.148 (dd 1H), 3.730-3.804 (dd, 1H), 5.518-5.480 (dd, 1H), 7.072-7.125 (dd, 3H), 7.283-7.372 (dd, 1H), 7.615 (dd, 1H), 7.634 (dd, 1H). HRMS: 368.1157. Anal. calcd for C18H15Cl2FN3O: C, 59.19; H, 4.14; Cl, 19.41; F, 5.20; N, 7.67; O, 4.38.
Candidate (3i)
White solid, yield: (302 mg, 75.39 %), m. p: 196±1°C; IR (cm-1): 3055(CO–CH3), 2927 (-CH3), 1695 (keto group C=O), 1585 (Pyrazoline C=N), 1515/1444 (Ar-ring C=C), 1329 (C-F), 1019 (N-N), 817 (C-Cl); 1H NMR (400 MHz, DMSO): d ppm 2.292 (s, 3H), 2.316 (s, 3H), 3.210-3.269 (m 1H), 3.838-3.914 (dd, 1H), 5.603-5.646 (dd, 1H), 7.235-7.309 (dd, 2H), 7.578-7.665 (dd, 2H,), 7.726-7.744 (dd, 1H). HRMS (m/z): 402.6042. Anal. calcd for C18H14Cl3FN3O: C, 54.09; H, 3.53; Cl, 26.61; F, 4.75; N, 7.01; O, 4.75.
Antimicrobial screening
The synthesized candidates 3(a-i) was in vitro tested for four bacterial strains (E. coli (MCC 2412), B. subtilis (MCC 2010), S. aureus (MCC 2408), Pseudomonas aeruginosa (MCC 2080)) and two fungal strains (Saccharomyces cerevisiae, MCC 1033, and Candida albicans, MCC 1439). Inhibition Zones and MIC values used to describe the antibacterial efficacy of the tested candidates 3(a-i). DMF served as a blank, while streptomycin and fluconazole served as the study’s reference drugs. The following approach was used to conduct the tests in triplicate.
Autoclaved Petri dishes were filled with sterilized bacterial (nutrient agar) and fungal (sabouraud dextrose agar) growth medium. In addition, 100 µl inocula of each test organism were swabbed onto the agar plates in a sterile environment. Adsorption was followed by creating pits of diameter (6mm) using a sterilised metallic borer and filling them with test sample solution (128 µg/20 µL). After 48 hours of incubation at 28 °C the ZOI and the MIC values for each chemical were determined using the broth double-dilution method with a 100 µl inoculums of each fungal culture 16, 17.
Results and discussion
Synthesis of chalcones and acylated pyrazolines compounds were synthesized with optimized reaction conditions with respect to time, temperature, reagent stoichiometry 14, 15.
The condensation of 4-fluoro-3-methylacetophenone (1) with cyano and chloro substituted benzaldehydes (a-i) carried place in presence of sodium hydroxide and ethanol throughout the synthesis of chalcones 2(a–i) according to Scheme-1. Pyrazoline derivatives 3(a–i) were synthesized through a cyclization reaction that involved chalcones 2(a–i) and hydrazine hydrate (Scheme 2) refluxed in the presence of glacial acetic acid.
FT-IR, 1H NMR and HRMS were used to characterize the novel heterocyclic chalcone candidates containing acylated pyrazoline 3(a–i). The presence of (carbonyl-C=O) and (carbon-nitrogen double bond–C=N) groups was determined from the FT-IR data of candidates 3(a–i), which displayed distinctive bands at around 1566–1630 cm-1 and 1652–1741 cm-1 18, 19. The FT-IR of the acylated pyrazoline 3(a–i) displayed distinctive bands with wavelengths ranging from 2924–2929 cm-1. These bands were developed by the C–H sp3 stretching in the –COCH3 group 20. A band at 1320-1363 cm-1 is indicates of C-F stretching aromatic ring. Bands at 1008-1037 cm-1 were observed in the acylated pyrazoline 3(a–i) indicates the presence of a (N-N) group. Absorption bands at 1501-1502 and 1400–1411 cm-1 were likewise present in candidates 3(a–i), indicating the presence of the C=C aromatic ring. Identifiable bands at 2227-2229cm-1 of -CN also observed in the IR spectrum of the candidates 3(a-c) 21. At a range of 817–821 cm-1, the infrared spectrum of the candidates 3(d–i) displayed a band that was characteristic of the stretching of the C-Cl group 22.
 |
Figure 1: FTIR data of 3b. |
The 1H NMR spectra of the candidates 3(a-i) confirmed their structures. Candidates 3(a–i) had a singlet of acyl group COCH3 protons at 2.300–2.360 dppm in their 1H NMR patterns. The singlet readings of three in high field region (d2.414–2.523 ppm) were found to be Ar-CH3 protons 23. Candidates 3(a-i) exhibited a pair of doublet-of-doublet resonances at 2.935-3.158 d ppm and 3.774-3.915 d ppm for the CH2 protons of the pyrazoline ring 23. In the range from 7.015-7.666 d ppm 22-25, multiplets set of five or six protons in the aromatic area were seen.
The mass spectrum confirmed the presence of M+ in the region, with a m/z measurement of 320.9945-398.2661, providing more evidence for the structure’s accuracy.
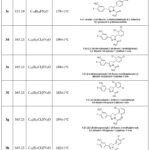 |
Table 2: Structures, melting points, and molecular weight of 3(a-i). |
Antibacterial activities
Table 3 indicated the antibacterial outcome of the investigated candidates 3(a-i) were evaluated against famous drugs (streptomycin) as internal standard comparing the zone diameters. It was found that candidates 3d and 3g showed greater inhibitory efficacy than remaining 3a, 3b, 3c, 3e, 3f, 3h, and 3i.
Table 3: Antibacterial studies of acylated pyrazoline 3(a-i)
|
Candidates |
Antibacterial Activity |
|||
|
S.-aureus |
B.-subtilis |
E.-coli |
P.-aeruginosa |
|
|
3a |
14 |
7 |
12 |
13 |
|
3b |
11 |
9 |
11 |
12 |
|
3c |
10 |
8 |
12 |
8 |
|
3d |
9 |
7 |
10 |
16 |
|
3e |
14 |
7 |
0 |
9 |
|
3f |
8 |
7 |
0 |
0 |
|
3g |
12 |
9 |
12 |
23 |
|
3h |
11 |
8 |
7 |
0 |
|
3i |
8 |
10 |
0 |
8 |
|
Streptomycin |
8 |
10 |
12 |
11 |
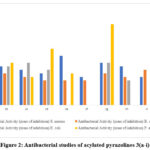 |
Figure 2: Antibacterial studies of acylated pyrazolines 3(a-i). |
Antifungal activity
For Candida albicans (MCC 1439), candidates 3c, 3d, and 3e showed the greatest inhibition, while candidates 3a, 3b, 3f, 3h, and 3i showed only moderate inhibition. Candidates 3c, 3d, and 3i demonstrated substantial inhibition against Saccharomyces cerevisiae (MCC 1033). To a lesser extent candidate 3a, 3b, 3f, and 3g inhibited Saccharomyces cerevisiae (MCC 1033) growth 26-29. Both fungal strains tested showed substantial action against chemicals 3c and 3d. Table 4 summarizes the results in detail.
Table 4: Antifungal activities of candidates 3(a-i)
|
Candidates |
Antifungal Activity (zone of inhibition/mm) |
|
|
C Albican |
S. C. |
|
|
3a |
8 |
8 |
|
3b |
9 |
6 |
|
3c |
12 |
12 |
|
3d |
10 |
11 |
|
3e |
0 |
11 |
|
3f |
8 |
6 |
|
3g |
7 |
20 |
|
3h |
0 |
6 |
|
3i |
0 |
7 |
|
Fluconazole |
11 |
10 |
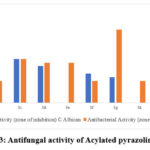 |
Figure 3: Antifungal activity of Acylated pyrazoline 3(a-i). |
Conclusion
The motive of this work is to expand the existing knowledge with the novel, useful candidates containing the acylated pyrazoline structure. To assess their in vitro biological activity, we have synthesized 9 novel acylated pyrazoline-derived candidates. The structures of these candidates were confirmed using FT-IR, NMR and HR-MS. The next step was to test the efficacy of the acylated pyrazolines 3(a-i) against a variety of bacteria, including Gram +ve (Bacillus subtilis MCC-2010 and Staphylococcus aureus MCC-2408) and Gram -ve (E. coli MCC-2412 and P aeruginosa MCC-2080) strains. The chemicals proved to be efficient against a wide variety of bacteria, and their antibacterial activity was even higher than that of streptomycin. The candidates 3c, 3d, 3g and 3e offer an initial point for new antifungal and antibacterial inhibitors.
Acknowledgment
The Savitribai Phule Pune University Maharashtra analytical support and Riva industries, Maharashtra for biological activity supports the authors are grateful.
Conflict of Interest
No conflict of interest.
References
- Goussault, H.; Salvator, H.; Catherinot, E.; Chabi, M. L; Tcherakian, C.; Chabrol, A.; Couderc, L. J. Respir. Res. 2019, 20:275, 1-10.
CrossRef - Yan-Li, Yang.; Zi-Jian, Xiang.; Jing-Hua, Yang.; Wen-Jie, Wang.; Zhi-Chun, Xu.; Ruo-Lan, Xiang. Front. Pharmacol, 2021, 12, 697330.
CrossRef - Charlier, C.; Hart, E.; Lefort, A.; Ribaud, P.; Dromer, F.; Denning, D. W.; Lortholary, O. J Antimicrob Chemother, 2006, 57 (3), 384-410.
CrossRef - Singh, V. K.; Chaurasia, H.; Mishra, R.; Srivastava, R.; Naaz, F.; Kumar, P.; Singh, R. K. J. Mol. Struct., 2022, 1247, 131400.
CrossRef - Karrouchi, K.; Radi, S.; Ramli, Y., Taoufik, J.; Mabkhot, Y. N.; Al-Aizari, F. A.; Ansar, M. H. Molecules, 2018, 23(1), 134.
CrossRef - Tantardini, C.; Arkhipov, S. G.; Cherkashina, K. A.; Kil’Met’Ev, A. S.; Boldyreva, E. V. Acta Crystallogr. E: Crystallogr., 2016, 72(12), 1856-1859.
CrossRef - Coro-Bermello, J.; Lopez-Rodríguez, E. R.; Alfonso-Ramos, J. E.; Alonso, D.; Ojeda-Carralero, G. M.; Prado, G. A.; Moreno-Castillo, E. Appl. Sci., 2021, 3, 1-20.
CrossRef - Owoyemi, B. C. D.; da Silva, C. C.; Diniz, L. F.; Souza, M. S.; Ellena, J.; Carneiro, R. L. CrystEngComm, 2019, 21(7), 1114-1121.
CrossRef - Cetin, A.; Bildirici, I. J. Saudi Chem. Soc., 2018, 22(3), 279-296.
CrossRef - Tripathi, A. C.; Upadhyay, S.; Paliwal, S.; Saraf, S. K. EXCLI J. 2018, 17, 126–148.
CrossRef - Marinescu, M. Antibiotics, 2021, 10 (8), 1002.
CrossRef - Anush, S. M.; Vishalakshi, B.; Kalluraya, B.; Manju, N. Int. J. Biol. Macromol., 2018, 119, 446-452.
CrossRef - Manna, K.; Banik, U.; Ghosh, P. S.; Das, M. Nirma Univ J Pharm Sci.; 2014, 1(1) 37-49.
CrossReff - Mohammed, Rayees, Ahmad.; Girija, V. Sastry.; Nasreen, Bano.; Syed, Anwar. Arab. J. Chem., 2016, 9 (1), S931-S935.
CrossRef - Xie, D.; Yang, J.; Niu, X.; Wang, Z.; Wu, Z. J Heterocycl Chem., 2022, 59(10), 1759-1767.
CrossRef - Kumar, A.; Rajeswara Rao, M.; Lee, W. Z.; Ravikanth, M. Org lett., 2017, 19(21), 5924-5927.
CrossRef - Pfaller, M. A.; Espinel-Ingroff, A.; Boyken, L.; Hollis, R. J.; Kroeger, J.; Messer, S. A.; Diekema, D. J. J. Clin. Microbiol., 2011, 49(3), 845-850.
CrossRef - Singh, V. K.; Mishra, R.; Kumari, P.; Som, A.; Yadav, A. K.; Ram, N. K.; Singh, R. K. Comput Biol Chem, 2022, 98, 107675.
CrossRef - Smith, B. C. Spectroscopy, 2017, 32(9), 31-36.
CrossRef - Ignatieva, L. N.; Beloliptsev, A. Y.; Kozlova, S. G.; Buznik, V. M. J. Struct. Chem., 2004, 45, 599-609.
CrossRef - Sajan, D.; Joe, I. H.; Jayakumar, V. S. J Raman Spectrosc. 2006, 37, 508–519.
CrossRef - Choi, S.; Park, J.; Kwak, K.; Cho, M. Chem. Asian J., 2021, 16 (18), 2626-2632.
CrossRef - Dozova, N.; Krim, L.; Alikhani, M. E.; Lacome, N. J. Phys. Chem.-A 2005, 109 (45), 10273-10279.
CrossRef - Nyquist, R.A. Academic Press. 2001, Vol 2.
CrossRef - Mullen, C. A.; Strahan, G. D.; Boateng, A. A. Energy & Fuels, 2009, 23(5), 2707-2718.
CrossRef - Mahajan, D. T.; Masand,V. H.; Patil, K.N.; Ben Hadda, T.; Jawarkar, R. D.; Thakur, S. D.; Rastija, V. Bioorg. Med. Chem. Lett. 2012, 22, 4827–4835.
CrossRef - Macêdo, W. J. C.; Braga, F. S.; Santos, C. F.; Costa, J. D. S.; de Melo, G. S.; de Mello, M. N.; dos Santos, C. B. R. J. Comput. Theor. Nanosci., 2015, 12 (10), 3443-3458.
CrossRef - Santos, C. B. R.; Vieira, J. B.; Lobato, C. C.; Hage-Melim, L. I.; Souto, R. N.; Lima, C. S.; Carvalho, J. C. T. Molecules, 2014, 19(1), 367-399.
CrossRef - Ibrahim, Z. Y. U.; Uzairu, A.; Shallangwa, G. A.; & Abechi, S. E. Iranian Journal of Pharmaceutical Research: IJPR, 2021, 20(3), 254.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









