Review on Various Antibiotic Contamination in Natural Sources: Effects on Environment Including Animals and Humans
Department of Chemistry, East Calcutta Girls’ College, Lake Town Link Road, Block B, Lake Town, Kolkata, West Bengal, India.
Corresponding Author E-mail: debashree1981@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400204
Article Received on : 17 Feb 2024
Article Accepted on :
Article Published : 29 Mar 2024
Reviewed by: Dr. Atamjit Singh
Second Review by: Dr. Ashok Akabari
Final Approval by: Dr. Abdelwahab Omri
Antibiotics have been used as medicine to inhibit a large array of infections in humans, in animals and plants for nearly 100 years. Nonstop use and misapplication of antibiotics have triggered antibiotic contamination worldwide. Antibiotic contamination poses risks to microbial communities, aquatic ecosystems, and human health. If growth of antibiotic resistant bacteria occurs, then it may increase multidrug-resistant bacterial infections for the coming days, thus posing a terrific impact on human health, as well as on the ecosystem of the environment. So, proper management and awareness are crucial to mitigate the environmental impacts. In this review, the knowledge about the sources and existence of antibiotics, its side effects, contamination sources, prevention and control of antibiotics to reduce antibiotic contamination has been discussed in detail.
KEYWORDS:Antibiotics; Contamination; Classification; Environment; Effects; Prevention
Download this article as:| Copy the following to cite this article: Mandal D. Review on Various Antibiotic Contamination in Natural Sources: Effects on Environment Including Animals and Humans. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Mandal D. Review on Various Antibiotic Contamination in Natural Sources: Effects on Environment Including Animals and Humans. Orient J Chem 2024;40(2). Available from: https://bit.ly/3TW3N3C |
Introduction
In pre 20th century it was common the death of people with minor infection. In 1928 first Alexander Fleming invented penicillin as antibiotic.1-3 It is used as medicine which can kill or stop the growth of bacteria. Before 1930, β-lactam was introduced whereas pleuromutilin4 was introduced. In 1941 Chain and Florey found out first the clinical use of antibiotics penicillin5 and got Nobel prize in 1945 for their work on antibiotic. Due to novel use of antibiotic, a great demand is developed and new antibiotics are discovered day by day.6,7,8 But due to inappropriate use and disposal of leftover antibiotic, it is kept today in environment contamination segment. Antibiotic contamination 9-15 in natural sources is a pressing concern of irreversible damage with far-reaching consequences for the natural ecosystems of environment, human health and healthcare practices. Antibiotics protect lives, but misuse of it may cause the growth and spread of antibiotic contamination which can promote the development of antibiotic resistance genes and drug-resistant bacteria.16-17 Concentrations of antibiotic residues in manure, sewage sludge, biosolids, and soil vary widely and spreads resistance through people by their food, contaminated meat, animal’s waste in the environment.18 Fruits, vegetables, and other food can become contaminated through touch with contaminated soil 19,20 or water containing waste from animals. Antibiotics are sometimes overused to make the animals21 grow faster which creates problem. Antibiotic uses also have been reported in aquaculture,22 beekeeping, and livestock as growth promoters. At the hatchery industry, eggs are generally injected with little amounts of an antibiotic called gentamicin, which is used in people to give a variation of serious bacterial infections.23 In human food in general these antibiotics are used or indirectly come from the egg and meat of animals: Tetracyclines, Subtilin,24 Tylosin, Nisin. In Tetracyclines family Chlorotetracycline (CTC) and Oxytetracycline (OTC) are used as food supplements as well as in fresh foods. On date, the most common antibiotic for safeguarding plants is Oxytetracycline and Streptomycin, but their use must be carefully managed to prevent the emergence of resistant pathogens.25 Subtilin is used in canned foods as well as an additive agent for detergent,26 as cleaner in laundry, in cosmetics, in contact lens cleaner etc. Tylosin exists in the macrolide antibiotic family and bacteriostatic in nature. It is used very much in animal feeds and also, it is used as growth promoter.27 The exposure of this antibiotic may create rash in human, that is why it is avoided.28 Nisin lies in the polypeptide antibiotic family. Recently it is used as preservatives in high moisture, processed food including dairy products, hot baked bakery products and pasteurized liquid egg.29 Beside this, all of we know, blood cultures are essential for diagnosing infections. Common sources of contamination include poor collection technique and insufficient skin disinfection. Contaminants often include coagulase-negative staphylococci, Corynebacterium spp., and other skin-related bacteria. Contaminated blood cultures can result in unnecessary antibiotic use, allergic reactions, and even Clostridioides difficile infection.30 Antibiotic consumption is also very crucial31 because contamination show residual effects on unnecessary antibiotic use.32
Classification of Antibiotics
To know more about antibiotic contamination, we should know before about the antibiotic. In the vast area of antibiotics, the drugs are classified (Fig. 1) in the following possible manner: 1) According to chemical structure of antibiotics33-34 as: i) Sulfonamides and related drugs: These are used for tonsillitis, pharyngitis, sinusitis, etc. e.g. Sulfanilamide,35 Sulfadiazine ii) Diaminopyrimidines36: It is used for the acute bacterial infection on skin. e.g. Trimethoprim, Pyrimethamine etc. iii) Quinolones37: . Urinary tract infection, typhoid fever are recovered by this drug. e.g. Norfloxacin, Ciprofloxacin, etc. Norfloxacin is another drug of this family which is used for urinary and genital tract infections and for orodental infections. iv) N-contained heterocyclic β-lactam antibiotics38,39: Penicillin, Cephalosporins are common example and is used in dentistry. v) O-contained heterocyclic antibiotics: These are used to treat tuberculosis40 but have some general side effects like allergic reactions, sleepiness, etc. e.g. Cycloserine: Oxygen- (sulphur- also) based heterocycles are gaining more attention in recent years41 on the road to the discovery of innovative anticancer drugs. vi) Tetracyclines42: e.g. Tetracycline, Oxytetracycline, etc. It is used to treat in some infections of the lungs, urinary tract, eyes etc. vii) Aromatic Nitrobenzene derivative: e.g. Chloramphenicol.43 It is used to treat eye conjunctivitis, meningitis, plague, cholera, and typhoid fever. viii) Carbohydrate contained Aminoglycosides antibiotic: These will have the core structure of carbohydrate.44 e.g. Streptomycin, Neomycin, Streptomycin, etc. It may cause misreading of m-RNA code and affect permeability.45 ix) Macrocyclic lactone/Macrolide antibiotics: e.g. Erythromycin, Azithromycin, etc. During COVID-19 pandemic period, due to health system disruptions, these antibiotic drugs were used most. As a result, the resistance of the antibiotic46 as well as cancer, urinary tract infection, and multi-drug-resistant tuberculosis patients increased worldwide.47-51 After effect of COVID-19 antibiotic use and its resistance also a horrible picture.52 x) Lincosamide antibiotics53: It is used to treat the Pneumonia infection, throat infection, stomach infection, skin and soft tissue’s infection, bones and joints infection, and teeth infection. e.g. Lincomycin, Clindamycin. xi) Polypeptide antibiotics54: e.g. Polymyxin-B, Bacitracin. It may cause kidney damage, but on gradually using these drugs is safe. xii) Glycopeptides55: Due to the toxicity of this drug, without emergency in general it is not used. e.g. Vancomycin xiii) Oxazolidinone56: It is used to cure severe bacterial infections of lungs (Pneumonia), skin and soft tissues. xiv) Nitrofuran derivatives57: Nitrofurantoin is used for the recovery of urinary tract infections. Common adverse effects are nausea, loss of appetite, diarrhea, headaches etc. xv) Nitroimidazoles58: e.g. Metronidazole, Tinidazole, etc. has some adverse effects like anorexia, nausea, bitter or metallic taste, as well as headache, glossitis, dryness of mouth. xvi) Nicotinic acid derivatives59: This drug is referred for tuberculosis. But the adverse effects are nausea, loss of appetite, muscle and joint pains, and rash may arise. e.g. Isoniazid, Pyrazinamide. xvii) Polyene antibiotics60,61: e.g. Nystatin, Amphotericin-B etc. This drug can be used topically for oral, vaginal and cutaneous candidiasis. xviii) Azole derivatives62: e.g. Miconazole, Clotrimazole, Ketoconazole, etc. Azole drugs interfere with DNA function and used in tonsillitis, pharyngitis, sinusitis, otitis media and orodental infections. xix) Others: Viomycin, Ethambutol, etc. This class of antibiotics are used for tuberculosis treatment.
 |
Figure 1: Classifications of Antibiotics in all possible way. |
Antibiotics according to Mechanism of action
Theaction mechanism63 generally occurred in the following way i) Inhibit cell wall synthesis/Peptidoglycan Inhibitors: e.g. Penicillin, Cephalosporins, etc. ii) Cause leakage from cell membranes: e.g. Polymyxins, Amphotericin B etc. iii) Inhibit protein synthesis: e.g. Tetracyclines, Streptomycin, Aminoglycosides, etc. iv) Cause misreading of m-RNA code and affect permeability: e.g. Aminoglycosides— Streptomycin, Gentamicin, etc. v) Inhibitor of Nucleic acid synthesis: e.g. Ciprofloxacin, Metronidazole, etc. vi) Inhibitor of folic acid synthesis (Folate antagonistic). e.g. Sulphonamide, Trimethoprim etc. vii) Inhibitor of cytoplasmic membrane. e.g. Polymyxin, Colistin etc.
Antibiotics according to Spectrum64: 1) Narrow spectrum: It is active towards relatively fewer microorganisms. e.g. macrol ides, Polymyxin Penicillin G, Streptomycin etc. 2) Moderate spectrum: Examples: Aminoglycosides, Sulphonamide etc. . 3) Narrow-Broad spectrum: Examples:-lactam. 4) Broad spectrum: e.g : Chloramphenicol, Tetracycline etc. This class of drug may arise epigastric pain, nausea, vomiting and diarrhea, acute hepatic necrosis may arise in pregnant women, risk of kidney damage, brown discoloration and ill-formed teeth.
Antibiotics according to Spectrum64
1) Narrow spectrum: It is active towards relatively fewer microorganisms. e.g. macrolides, Polymyxin Penicillin G, Streptomycin etc. 2) Moderate spectrum: Examples: Aminoglycosides, Sulphonamide. 3) Narrow-Broad spectrum: Examples: β-lactam. 4) Broad spectrum: Examples: Chloramphenicol, Tetracycline. This class of drug may arise epigastric pain, nausea, vomiting and diarrhea, acute hepatic necrosis may arise in pregnant women, risk of kidney damage, brown discoloration, ill-formed teeth.
Antibiotics according to Action65,66
These are divided as i) bactericidal (Antibacterials, which destroy bacteria by targeting the cell wall or cell membrane of the bacteria. e.g. Tetracyclines, Macrolides, Spectinomycin, Chloramphenicol etc.) and ii) bacteriostatic (Antibacterials that slow or inhibit the growth of bacteria. e.g. Penicillin, Cephalosporins, Fluoroquinolones etc.) where former is preferred over bacteriostatic.
Antibiotics according to source
Type of organisms against which primarily energetic: 1) Microbial origin antibiotics: three type of origin has been seeni) Antibacterial: e.g. Penicillin, Aminoglycosides, etc. ii) Antifungal: e.g. Amphotericin B, Ketoconazole etc. iii) Actinomycetes: e.g. Streptomycin, Chloramphenicol etc. iv) Antiviral: e.g. Acyclovir, Amantadine, etc. v) Antiprotozoal: e.g. Chloroquine, Metronidazole, etc. vi) Anthelmintic: e.g. Mebendazole, Niclosamide, etc. 2) Semi-synthetic antibiotics67: e.g. Amoxycillin, Sulfonamide etc. 3) Synthetic antibiotics68: e.g. 4-quinolones, Sulfonamide etc.
Antibiotics according to the basis of Route of administration69:
The severity of infection is seen for antibiotics administration. a) Oral antibiotics: These antibiotics are taken orally. e.g. Penicillin V.b) Parenteral route: These antibiotics are taken by intravenous injection. e.g. Penicillin G.
The Sources70 of antibiotic contamination
It has seen71 that the primary sources of antibiotic contamination are due to antibiotic use in human medicine, veterinary medicine and agriculture. Addressing antibiotic contamination remains a critical challenge for environmental sustainability.70a,72-75 The contamination of aquatic environments by antibiotics which are widely used in medicine and agriculture, end up in wastewater involves several interconnected mechanisms (Fig. 2): a) Human and animal excretion : Antibiotics are consumed by humans and animal to treat infections or as feed additives. When humans and animals consume antibiotics, their bodies partially metabolize these drugs. However, a significant portion remains unchanged and is excreted via urine and feces. These residues enter sewage systems and eventually reach wastewater treatment plants.70a b) Wastewater treatment plants: Most antibiotics are not fully metabolized in the body because they are often non-biodegradable. These residues enter into sewage systems and wastewater treatment plants (WWTP) either unchanged or as metabolites. c) Inadequate removal in conventional WWTP effluents:Conventional WWTPs are ineffectiveat completely removing antibiotics because they are not designed to effectively eliminate antibiotics and their resistance genes. As a result, while some degradation occurs, a substantial amount of antibiotics and their by-products persists in the treated wastewater effluents.72 d) Release into the aquatic environment: These effluents are then discharged into water bodies, such as rivers, lakes, and oceans. Antibiotics, along with their metabolites, are released into these water bodies, persist in the environment, posing a contamination75 to aquatic ecosystems. e) Adsorption and accumulation: Antibiotics are adsorbed onto suspended particles, sediments, and organic matter in water. They can accumulate in aquatic ecosystems over time, especially in areas with slow water flow f) Antibiotic-resistant bacteria (ARB): The presence of antibiotics in water resources leads to the emergence of antibiotic-resistant bacteria (ARB). These types of resistant bacteria possess resistance genes survive and multiply, leading to the emergence of antibiotic-resistant strains. These resistant bacteria can then spread to other organisms in the environment. g) Human and animal exposure: Unused antibiotics and antibiotic resistant bacteria can then spread through different sections of the environment (e.g. surface water, groundwater, drinking water, municipal sewage, soil, vegetables, sludge etc.), subsequently rising in antibiotic resistance and affecting human and animal health.
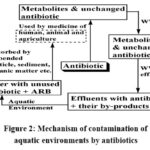 |
Figure 2: Mechanism of contamination of aquatic environments by antibiotics |
There are several sources also which contribute antibiotic contamination to the presence of antibiotics in the environment. i) Pharmaceutical manufacturing waste: The production of antibiotics generates waste, which can contain residual antibiotics. If not properly managed, this waste can contaminate water sources and contribute to the spread of antibiotic resistance. ii) Improper disposal of unused medication: When people dispose of unused or expired antibiotics, they may end up in landfills or leach into the environment. This improper disposal can introduce antibiotics into soil and water systems. iii) Wastewater from hospitals and municipalities: Hospitals and municipalities release wastewater containing antibiotics. Conventional wastewater treatment plants are often unable to effectively remove antibiotics and their resistance genes which permanently stay in the environment. iv) Large-scale animal farms and aquaculture: Antibiotics are commonly used in animal farming and aquaculture. It plays a significant role in environmental contamination, with far-reaching consequences. There are several steps how this contamination occur9,76: a) Waste disposal and manure: Large-scale animal farms, also known as concentrated animal feeding operations, house a substantial number of animals. These animals are often given antibiotics to prevent or treat infections. The unmindful release of antibiotics occurs through the disposal of animal waste. When animals excrete antibiotics or their metabolites, these compounds end up in manure and are spread on fields as fertilizer. This practice introduces antibiotics into the soil and nearby water bodies. b) Surface runoff and leaching: Rainwater can wash antibiotic-laden manure from fields into nearby streams, rivers, and groundwater. This surface runoff and leaching transport antibiotics and their residues into aquatic environments. As a result, water bodies become contaminated with these compounds. c) Aquaculture and fish farming: Antibiotics are also used in aquaculture (fish and shrimp farming). In these systems, antibiotics are added to fish feed to prevent diseases. However, excess antibiotics can leach into surrounding water bodies, leading to contamination. Aquatic ecosystems become reservoirs for antibiotic residues and resistant bacteria. d) Antibiotic resistance: The presence of antibiotics in the environment exerts selective pressure on bacteria. Those bacteria that possess resistance genes survive and multiply. Over time, this contributes to the emergence of antibiotic-resistant strains. These resistant bacteria can spread to other organisms, including humans, through water, soil, and food chains. e) Ecological impact: Antibiotics can disrupt natural microbial communities in soil and water. This disturbance affects nutrient cycling, ecosystem stability, and overall biodiversity. Additionally, non-target organisms (such as aquatic insects, algae, and fish) may be harmed by exposure to antibiotic residues. f) Human health concerns: Antibiotic-contaminated water can directly or indirectly affect human health. Consuming water or food with traces of antibiotics may contribute to the development of antibiotic resistance in human pathogens. Moreover, antibiotic-resistant bacteria can transfer their resistance genes to human pathogens, reducing the effectiveness of medical treatments. As a result of this antibiotic contamination the following consequences are seen: 1) Antibiotic resistance: The proliferation of antibiotic-resistant bacteria in the environment poses a serious threat to public health. These resistant strains can cause difficult-to-treat infections in humans and animals. 2) Ecological disruption: Antibiotics alter microbial communities, affecting nutrient cycling and ecosystem dynamics which affects other organisms. 3) Water quality: Antibiotic contamination reduces water quality, impacting marine lifecycle and potentially disturbing drinking water foundations. 4) Food safety: If antibiotic-contaminated water is used for irrigation or aquaculture, it can lead to the presence of antibiotics in crops and seafood, posing risks to consumers. v) Industrial and Agricultural emissions: Chemical pollution from industrial and agricultural activities can also introduce antibiotics into surface waterways, posing a serious threat to environmental sustainability and a high ecologicalimpact on the environment. The journey of antibiotics from human consumption to wastewater treatment and eventual release into water bodies contributes to antibiotic contamination and poses a serious threat to environmental and human well-being.1)Aquatic ecosystems: Antibiotics disrupt natural microbial communities in water bodies. This disturbance affects nutrient cycling, food webs, and overall ecosystem health. The accumulation of antibiotics in aquatic environments can have long-term consequences, including the development of antibiotic-resistant bacteria, leading to a loss of species diversity, and potential harm to aquatic organisms. Commonly found antibiotics include sulfonamides, macrolides, and quinolones.2)Human health: Exposure to antibiotics in water resources can lead to the emergence of antibiotic-resistant bacteria. These resistant strains pose a risk to human health. So proper treatment of water before discharge is crucial to prevent the spread of antibiotic contamination. In this situation, people would think about the use of natural alternative antibiotics which do not contribute to resistance.
Side effects of Antibiotic77,78
Antibiotics are used in treating severe bacterial infections, can also have side effects such as a) Gastrointestinal Distress: Nausea, vomiting (e.g. Trimethoprim-Sulfamethoxazole) and diarrhea (e.g. Clindamycin, Amoxicillin etc.) are common side effects. Some antibiotics can disrupt the balance of gut bacteria, leading to gastrointestinal discomfort (e.g. Azithromycin, Doxycycline etc.). b) Allergic Reactions: Rash, itching, and hives (e.g. Trimethoprim-Sulfamethoxazole, penicillin etc.) may occur. In severe cases, anaphylaxis (a life-threatening allergic reaction) can happen. c) Photosensitivity: Certain antibiotics (e.g. Ciprofloxacin) make your skin more sensitive to sunlight. Sunburn, rashes, or skin discoloration may result from sun exposure. These can close the heart rhythm. d) Liver Toxicity: Some antibiotics can affect liver function (e.g. Flucloxacillin). Jaundice (e.g. Augmentin) is a sign of liver involvement. e) Nephrotoxicity: Vancomycin can cause nephrotoxicity. f) Kidney Damage: Prolonged use of certain antibiotics can harm the kidneys (e.g. Gentamicin, Streptomycin, Trimethoprim, Sulfamethoxazole etc.). Kidney pain, changes in urine color, or reduced urine output should be monitored. g) Tendon Rupture: Fluoroquinolone antibiotics (e.g. ciprofloxacin) carry a risk of tendon rupture. Avoid strenuous physical activity if you experience tendon pain. h) Super infections: Antibiotics can disrupt the normal balance of microbes. This may lead to yeast infections, oral thrush, or other secondary infections. (e.g. cephalosporins, fluoroquinolones etc.) i) Drug Interactions: Some antibiotics interact with other medications. Always inform your healthcare provider about all the drugs you’re taking. j) Candida Overgrowth: Prolonged antibiotic (e.g. Nystatin, Amphoterin B etc.) use can lead to an overgrowth of Candida (a type of yeast). It (Fluconazole, itraconazole, and voriconazole etc.) may cause oral thrush or vaginal yeast infections. k) Development of Antibiotic Resistance and Gut: Resistance always arises by three mechanis79 steps: 1) natural resistance80 in certain types of bacteria, 2) genetic mutation, 3) one species acquiring resistance from another,81 is increasing day by day because i) limited supportive care provided by health professionals. ii) difficulty in treating various infections iii) antibiotic Resistance – Economic burden to the healthcare system iv) increasing population.82 This resistance is one of the major terrorizations to global health, food security83 today. Gut is developing in human beings through food. People with gut usually do not need antibiotics84,85 to get better. People with severe infections antibiotics may essential, otherwise people may at risk86 for serious complications87 include infants and old person.
Prevention and control of the antibiotic contamination
To reduce the spreading of antibiotic resistance, we should aware88,89 some prescribed rules: I) Patients should stop90 the taking of improper of drug and doses. People should follow the WHO91 Five Keys to Safer Food, avoiding close contact with sick people. II) Policymakers should control the spread of antibiotic resistance, by the following steps92: i) Enforcement of regulations: Policymakers play a vital role in enforcing lawsand regulations related to antibiotic use; a) Restrict non-prescription antibiotic sales: Ensuring that antibiotics are only used under the guidance of trained healthcare providers. b) Monitor and regulate antibiotic production: Implementing standards for pharmaceutical manufacturers to minimize contamination during production. c) Promoting Sustainable Practices: Policymakers can incentivize sustainable practices in agriculture, aquaculture, and waste management to reduce antibiotic contamination. d) Supporting Research and Surveillance: Funding research on antibiotic contamination and supporting surveillance programs to monitor resistance patterns and environmental contamination.93 III) Healthcare Professionals also must obey some rules75: i) Appropriate Prescribing Practices: Healthcare professionals should a) Prescribe antibiotics judiciously: Avoid unnecessary prescriptions and choose narrow-spectrum antibiotics when possible, b) Educate patients: Raise awareness about responsible antibiotic use and the risks of contamination, c) Infection Prevention and Control (IPC): Implement IPC measures to prevent the spread of resistant bacteria in healthcare settings, and d) Reporting and Surveillance: Healthcare professionals can report cases of antibiotic-resistant infections and contribute to surveillance efforts. Veterinary professionals should be aware not to use antibiotics for growth promotion and work closely with farmers to ensure proper antibiotic use in animals. IV) Agriculture sector should follow the following steps to reduce contamination94: i) Responsible antibiotic use in livestock and aquaculture: a) Withdrawal Periods: Implement appropriate withdrawal periods to prevent antibiotic residues in animal products. ii) Waste management: a) Manure Handling: Properly manage animal waste to prevent antibiotic runoff into soil and water. b) Crop Fields andAquaculture: Implement standards for treating and managing discharge from farms. iii) Sustainable Farming Practices: a) Reduce antibiotic dependency: Explore alternatives to antibiotics, such as probiotics and vaccines. b) Integrated pest management: Use practices that minimize the need for antibiotics. c)Crop rotation and soil Health: Maintain healthy soil ecosystems to reduce the need for antimicrobial. d) Organic food production: promote about the growing of organic food. V) Water should be antibiotic free95,96 Huge antibiotics are consumed by humans and animals’ excretion via urine and goes into sewage systems and treatment plants. VI) The dominant antibiotic contaminants include sulfamethazine, trimethoprim, oxolinic acid, ciprofloxacin, and others should be analyzed and prevalence of antibiotic resistance genes is essential.97 VII) People has lack of knowledge of effects and resistance of antibiotic.98 Some prevention we should take to control the antibiotic resistance by 1) choice of Antibiotic and 2) drug factor is important. Less toxic antibiotic is preferred because it impacts on central nervous system.99 So, we are looking for the natural path of antibiotic treatment from all researchers now100 to reduce resistance. Otherwise, antibiotic would affect ecology system.15,101So, we can take some preventive measures to control antibiotic contamination based on relevant sources102: i) Standard Precautions for controlling antibiotic contamination: a) Risk Assessment: Health workers should assess the risk of exposure to blood, body fluids, and contaminated surfaces. Prevention measures should be based on this assessment. b) Hand Hygiene: Promote handwashing with soap or provide alcohol-based hand rub at the point of care. c) Personal Protective Equipment (PPE): Train health workers on correct PPE use. Ensure continuous access to high-quality PPE. d) Respiratory Hygiene and Cough Etiquette: Display visual alerts for respiratory hygiene. Provide hand hygiene supplies and masks in waiting areas. e) Environmental Cleaning: Maintain a clean and hygienic environment. Train cleaning staff on proper practices and disinfection products. ii) Environmental Health Practices to Prevent Antimicrobial Resistance (AMR): a) Water, Sanitation, and Hygiene (WASH): 1) Ensure access to safe water. 2) Properly contain, treat, and dispose of human excreta and wastewater. 3) Promote personal hygiene practices (e.g. handwashing) to prevent the spread of resistant microorganisms. b) Solid Waste Disposal: Dispose of unused and expired antimicrobial properly to prevent unnecessary exposure in the environment. c) Food Hygiene and Safety: 1) Ensure adequate antimicrobial withdrawal periods for animal products. 2) Grow vegetables on unpolluted soil. iii) Contamination Control Strategies: a) Risk Assessment: Understand the sterile product manufacturing process. Assess process variables and sources of contamination. b) Acceptance Criteria and Metrics: Set achievable criteria for contamination prevention. c) Monitoring and Adjustment: Continuously monitor performance and adjust strategies as needed. These measures emphasize responsible antibiotic use, proper waste management, and hygiene practices to safeguard both human health and the environment. Antibiotic contamination generally removed by either waste water treatment (ultrafiltration,microfiltration, nanofiltration, membranous biological reactor treatment) or using alternatives of antibiotics. These membrane-based techniques can effectively remove antibiotics. Combining biological processes with membranes enhances removal efficiency. Advanced Oxidation Process (AOP; AOPs break down antibiotics using chemical reactions), Reverse Osmosis (RO; RO membranes remove antibiotics) and Nano sorbents(Nanomaterials can adsorb antibiotics effectively) etc.70a,96 Another way is to take alternatives to antibiotics, if there is no severe infection. There are several alternative antibiotic therapy103-106: i) Probiotics: Probiotics are live microorganisms (usually bacteria or yeast) that provide health benefits when consumed in adequate amounts. The mechanism of action of probiotic are as follows: a) Probiotics can restore and maintain gut microbial balance. b) By colonizing the gut, they compete with harmful bacteria, preventing their growth. c) Some probiotics produce antimicrobial compounds that inhibit pathogenic bacteria. It has immense impact on Antibiotic Resistance and Contamination: 1) Probiotics can enhance our immune system and compete with harmful bacteria, reducing the need for antibiotics by promoting overall health and preventing infections. 2) When used alongside antibiotics, probiotics may help minimize antibiotic-associated diarrhea and maintain gut health. 3) By supporting the gut microbiome, probiotics indirectly contribute to reducing antibiotic resistance. Probiotics are commonly found in yogurt, kefir, and dietary supplements. ii) Phage Therapy: Phage therapy involves using bacteriophages (viruses that infect bacteria) to treat bacterial infections. The mechanism of action of this therapy are as follows: a) Bacteriophages specifically target and infect specific bacterial strains. b) They replicate within the bacterial host, leading to its destruction. c) Phages do not harm human cells. This therapy also has good impact on antibiotic resistance and contamination: 1) Phage therapy offers an alternative to antibiotics, especially when bacteria are resistant to multiple drugs. 2) It can target specific pathogens, reducing the risk of resistance. 3) Phages are environmentally friendly and do not persist in the ecosystem. Phage therapy is still in research and development but holds promise for combating antibiotic-resistant infections. iii) Antiseptics and Disinfectants: Antiseptics (like hydrogen peroxide or iodine) and disinfectants (such as alcohol) can help prevent infection without relying on antibiotics only for external use. iv) Herbal Remedies: Some herbs and plants have natural antimicrobial properties. e.g. garlic, honey, oregano oil, and tea tree oil. v) Silver Nanoparticles: It has shown antibacterial properties and can be used as an alternative to antibiotics.107 vi) Immune-Boosting Foods and Supplements: a) Consuming foods rich in vitamins (especially vitamin C and zinc) can strengthen the immune system. b) Supplements like echinacea and elderberry may also help. vii) Limiting Antibiotic Use: Proper hygiene, vaccination, and avoiding unnecessary antibiotic prescriptions can reduce reliance on antibiotics. The choice of alternatives depend on the specific situation, type of infection, individual health, and consultation of healthcare professionals for personalized advice.
Conclusions
Antibiotic use is the strong weapon in these days. So, public (included general practitioner, pharmacist and patient also) should be concern about the use of antibiotic correctly and carefully. As research is going on the control of diseases, the use of antibiotics in the future will undoubtedly change. But we can use the alternative antibiotics if severe situation does not arise. A collaborative and constant efforts involving policymakers, healthcare professionals, in the agricultural sector, and alternative medicines by experts are to be developed. Natural antimicrobial108 may be introduced in food against multidrug-resistant bacterial infections’ therapy. Antibiotic contamination should be addressed in various sectors, awaking people of its in-control use, proper responsible disposal practices, enforcing regulations, and vigorous wastewater treatment to protect the health of the environment and humans. Everyone should remember to take antibiotics as prescribed, complete the full course, and discuss with doctors if any severe side effects arise.
Acknowledgement
Author would like to acknowledge National Library of Medicine and Wikipedia from where she had collected some information about Antibiotic from online.
Conflict of Interest
The author declares no conflict of interest regarding the publication of this review article.
Funding Sources
There is no funding sources
References
- Letek, M. Frontiers for Young Minds, 2020, 7, 159-165. doi:10.3389/fr ym.2019.00159
- Bhattacharjee, M. K. Chemistry of Antibiotics and Related Drugs Book from Springer 2022, second Edition, 1-11. https:// doi.org/10.1007/978-3-031-07582-7_1
- Hutchings, M. I.; Truman, A. W.; Wilkinson, B. Current Opinion in Microbiology, 2019, 51, 72-80. https://doi.org/10.1016/j.mib.2019.10.008.
- Goethe, O.; DiBello, M.; Herzon, S. B. Nat. Chem. 2022, 14, 1270–1277. https://doi.org/10.1038/s41557-022-01027-7
- Menard, D.; Dondorp, A. Cold Spring Harb. Perspect. Med. 2017, 7(7), a025619- a025643. doi: 10.1101/cshperspect.a025619.
- Rawson, T. M.; Wilson, R. C.; Moore, L. S. P.; Macgowan, A. P.; Lovering, A. M.; Bayliss, M.; Kyriakides, M.; Gilchrist, M.; Roberts, J. A.; Hope, W. W.; Holmes, A. H. Open Forum Infectious Diseases, 2021, 8(12), 1-4. doi:10.1093/ofid/ofab573
- Al-Tawfiq, J. A.; Momattin, H.; Al-Ali, A. Y.; Eljaaly, K.; Tirupathi, R.; Haradwala, M. B.; Areti, S.; Alhumaid, S.; Rabaan, A. A.; Al Mutair, A.; Schlagenhauf, P. Infection 2022, 50(3), 553-564. doi: 10.1007/s15010-021-01709-3
- Klein, E. Y.; Van Boeckel, T. P.; Martinez, E. M.; Pant, S.; Gandra, S.; Levin, S. A.; Goossens, H.; Laxminarayan, R. Proc. Natl. Acad. Sci. 2018, 115(15), E3463-E3470. doi: 10.1073/pnas.1717295115.
- Bombaywala, S.; Mandpe, A.; Paliya, S.; Kumar, S. Environ. Sci. Pollut. Res. Int. 2021, 28(20), 24889-24916. doi: 10.1007/s11356-021-13143-x.
- Polianciuc, S. I.; Gurzău, A. E.; Kiss B.; Ştefan M. G.; Loghin F. Med Pharm Rep. 2020, 93(3), 231-240. doi: 10.15386/mpr-1742.
- Gothwal, R.; Shashidhar, T. CLEAN – Soil, Air, Water, 2015, 43(4), 479-489. doi: org/10.1002/clen.201300989
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M. H.; Zheng, C. Environmental Research, 2019, 169, 483-493. https://doi.org/10.1016/j.envres. 2018.11.040.
- Larsson D. G. J.; Flach C. F. Nat. Rev. Microbiol. 2022, 20(5), 257-269. doi: 10.1038/s41579-021-00649-x.
- Singer, A. C.; Shaw, H.; Rhodes, V.; Hart, A. Front Microbiol. 2016, 7, 1728-50. doi: 10.3389/fmicb.2016.01728.
- Gangar, T.; Patra, S. 3 Biotech. 2023, 13(12), 401-423. doi: 10.1007/s13205-023-03806-6.
- Kumar, S. B.; Arnipalli, S. R.; Ziouzenkova, O. Antibiotics (Basel) 2020, 9(10), 688-704. doi: 10.3390/antibiotics9100688.
- Giacometti, F.; Shirzad-Aski, H.; Ferreira, S. Antibiotics. 2021, 10(6), 671-700. doi: org/10.3390/antibiotics10060671
- Larsson, D. G. J.; Flach, C. F. Nat Rev Microbiol. 2022, 20(5), 257-269. doi: 10.1038/s41579-021-00649-x.
- Kourkouta, L.; Kotsiftopoulos, C.; Papageorgiou, M.; Iliadis, C.; Monios, A., J. Healthcare Commun. 2017, 2, 1-4. doi: 10.4172/2472-1654.100076
- Mann, A.; Nehra, K.; Rana, J. S.; Dahiya, T. Curr. Res. Microb. Sci. 2021, 2, 100030-44. doi: 10.1016/j.crmicr.2021.100030.
- Schar, D.; Sommanustweechai, A.; Laxminarayan, R.; Tangcharoensathien, V. PLoS Medicine 2018, 15(3), e1002521-30. doi: 10.1371/journal.pmed.1002521
- Sharma, L.; Siedlewicz, G.; Pazdro, K. Plants 2021, 10(3), 591-606. doi: org/10.3390/plants10030591
- Hathorn, E.; Dhasmana, D.; Duley, L.; Ross, J. D. Syst. Rev. 2014, 3, 104-112. doi: 10.1186/2046-4053-3-104
- Azrin, N. A. M.; Ali, M. S. M.; Rahman, R. N. Z. R. A.; Oslan, S. N.; Noor, N. D. M. Biotechnol. Appl. Biochem. 2022, 69(6), 2599-2616. doi: 10.1002/bab.2309.
- Verhaegen, M.; Bergot, T.; Liebana, E.; Stancanelli, G.; Streissl, F.; Mingeot-Leclercq, M. P.; Mahillon, J.; Bragard, C. Front. Microbiol. 2023, 14, 1221478-95. doi: 10.3389/fmicb.2023.1221478.
- Gulmez, C.; Atakisi, O.; Dalginli, K.Y.; Atakisi, E. Int. J. Biological Macromolecules 2018, 108, 436-443. doi: org/10.1016/j.ijbiomac.2017.11.133.
- The international encyclopedia of Adverse Drug Reactions and interactions, Tylosin, Meyler’s side effects of drugs (sixteenth edition), 2016; pp. 233.
- Cazer, C.L.; Eldermire, E. R. B.; Lhermie, G.; Murray, S. A.; Scott, H. M.; Gröhn, Y. T. Prev. Vet. Med. 2020, 176, 104934-104953. doi: 10.1016/j.prevetmed.2020.104934.
- Shin, J. M.; Gwak, J. W.; Kamarajan, P.; Fenno, J. C.; Rickard, A. H.; Kapila, Y. L. J. Appl. Microbiol. 2016, 120(6), 1449-1465. doi: 10.1111/jam.13033.
- Clinical Practice Guideline: Prevention of Blood Culture Contamination. J. Emerg. Nurs. 2018, 44(3), 285.e1-285.e24. doi: 10.1016/j.jen.2018.03.019. Epub 2018 May 8. PMID: 29784085.
- Bloomfield, M. G.; O’Connor, M. J. Q.; Balm, M. N. D.; Blackmore, T. K. Open Forum Infectious Diseases 2022, 9(10), ofac529-ofac533. https://doi.org/10.1093/ofid/ofac529
- Chen, J.; Ying, G-G.; Deng, W-J. J. Agric. Food Chem. 2019, 67, 27, 7569–7586.
- Etebu, E.; Arikekpar, I. Int. J. Appl. Microbio. Biotech. Research. 2016, 4, 90-101. ISSN 2053-1818. https://www.researchgate.net/publication/319881509
- Mendes, C. d. d. S.; Antunes, A. M. d. S. Antibiotics (Basel) 2013, 2(4), 500-534. doi: 10.3390/antibiotics2040500.
- Nunes, O. C.; Manaia, C. M.; Kolvenbach, B. A.; Corvini, P. F. Appl. Microbiol. Biotechnol. 2020, 104(24), 10389-10408. doi: 10.1007/s00253-020-10982-5.
- Wróbel, A.; Arciszewska, K.; Maliszewski, D.; Drozdowska, D. J. Antibiot. 2020, 73, 5–27. https://doi.org/10.1038/s41429-019-0240-6
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Molecules 2020, 25, 5662-5689. https://doi.org/10.3390/molecules25235662
- Eckburg, P.B.; Lister, T.; Walpole, S.; Keutzer, T.; Utley, L.; Tomayko, J.; Kopp, E.; Farinola, N.; Coleman, S. Antimicrob. Agents Chemother. 2019, 63(9), e00892-19. doi: 10.1128/AAC.00892-19
- Lima, L. M.; Silva, B. N. M. D.; Barbosa, G.; Barreiro E. J. Eur. J. Med. Chem. 2020, 208, 112829-901. doi: 10.1016/j.ejmech.2020.112829.
- Wang, J.; Pang, Y.; Jing, W.; Chen, W.; Guo, R.; Han, X.; Wu, L.; Yang, G.; Yang, K.; Chen, C.; Jiang, L.; Cai, C.; Dou, Z.; Diao, L.; Pan, H.; Wang, J.; Du, F.; Xu, T.; Wang, L.; Li, R.; Chu, N. Infect. Drug. Resist. 2019, 12, 763-770. doi: 10.2147/IDR.S194484.
- Singh, P. K.; Silakari, O. Chem. Med. Chem. 2018, 13(11), 1071-1087.
- García-Fernández, A.; Dionisi, A. M.; Arena, S.; Iglesias-Torrens, Y.; Carattoli, A.; Luzzi, I. Front. Microbiol. 2018, 9, 1906-1914. doi: 10.3389/fmicb.2018.01906.
- Dinos, G. P.; Athanassopoulos, C. M.; Missiri, D. A.; Giannopoulou, P. C.; Vlachogiannis, I. A.; Papadopoulos, G. E.; Papaioannou, D.; Kalpaxis, D. L. Antibiotics 2016; 5(2), 20-41. doi: org/10.3390/antibiotics5020020
- Silva, R.; Coelho, E.; Aguiar, T. Q.; Domingues, L. Appl. Sci. 2021, 11, 8500-8522. https://doi.org/10.3390/app11188500
- G. S. Singh, Chapter Thirteen – Carbohydrate-based antibiotics: Opportunities and challenges, Editor(s): Vinod Kumar Tiwari, Carbohydrates in Drug Discovery and Development, Elsevier, 2020, Pages 523-559, ISBN 9780128166758, https://doi.org/10.1016/B978-0-12-816675-8.00013-0.
- Toro-Alzate, L.; Hofstraat, K.; de Vries, D. H. Int. J. Environ. Res. Public Health 2021, 18(16), 8766-80. doi: 10.3390/ijerph18168766.
- Arshad, M.; Mahmood, S. F.; Khan, M.; Hasan, R. BMJ. 2020, 371, m4501-m4503. doi:10.1136/bmj.m4501.
- Olamijuwon, E.; Keenan, K.; Mushi, M. F.; Kansiime, C.; Konje, E. T.; Kesby, M.; Neema S.; Asiimwe B.; Mshana, S. E.; Fredricks, K. J.; Sunday, B.; Bazira J.; Sandeman, A.; Sloan, D. J.; Mwanga, J. R.; Sabiiti, W.; Holden, M. T. G. J. Global Health 2024, 14, 05007-05017. doi:10.7189/jogh.14.05007.
- Huang, Z.; Tay, E.; Kuan, W. S.; Tiah, L.; Weng, Y.; Tan, H. Y.; Seow, E.; Peng, L. L.; Chow, A. Antimicrob. Resist. Infect. Control 2023, 12(1), 54-70. doi: 10.1186/s13756-023-01255-7.
- a) Jainlabdin, M. H.; Daleena, N.; Zainuddin, M.; Afiqah, S.; Ghazali, M. International Journal of Care Scholars. 2021, 4(2), 30-39, doi:10.31436/ijcs.v4i2.196 b) McCullough, A. R.; Rathbone, J.; Parekh, S.; Hoffmann, T. C.; Del Mer, C. B. J. Antimicrob. Chemother. 2015, 70(9), 2465-73. doi: 10.1093/jac/dkv164.
- Olamijuwon, E. O.; Konje, E. T.; Kansiime, C.; Kesby, M.; Keenan, K.; Neema, S.; Asiimwe, B.; Mshana, S. E.; Mushi, M. F.; Loza, O.; Sunday, B.; Sandeman, A.; Sloan, D. J.; Benitez-Paez, F.; Mwanga, J. R.; Sabiiti, W.; Holden, M. T. G.; Christine, J.; Kathryn, I.; Bazira, J.; Fredricks, M. M.; Bazira, J.; Muhumuza, C.; Muhwezi, I.; Fredricks, K. J. Antimicrobial Resistance & Infection Control 2024, 12(1), 10-24. doi:10.1186/s13756-022-01199-4
- Dey, P.; Parai, D.; Hossain, S. T.; Mukherjee, S. K. Universitas Scientiarum 2023, 28(2), 183-199. doi: 10.11144/Javeriana.SC282.taoc
- Meyler’s Side Effects of Drugs (Sixteenth Edition). The International Encyclopedia of Adverse Drug Reactions and Interactions 2016, 16, 581-588. doi: org/10.1016/B978-0-444-53717-1.00980-X
- Nayab, S.; Aslam, M. A.; Rahman, S. U.; Sindhu, Z.; Sajid, S.; Zafar, N.; Razaq M.; Kanwar, A. R. Int. J. Peptide Research and Therapeutics 2022, 28, 1-15. doi: org/10.1007/s10989-021-10325-6
- Butler, M. S.; Hansford, K. A.; Blaskovich, M. A.; Halai, R.; Cooper, M. A. J. Antibiot. (Tokyo) 2014, 67(9), 631-44. doi: 10.1038/ja.2014.111.
- Foti, C.; Piperno, A.; Scala, A.; Giuffrè, O. Molecules 2021, 26(14), 4280-93. doi: 10.3390/molecules26144280.
- Leyva-Aizpuru, A. P.; Quezada-García, Y. A.; Ramirez-Alonso, G.; Hinojos-Gallardo L. C.; Camarillo-Cisneros, J. (2023). Nitrofuran Antibiotics and Their Derivatives: A Computational Chemistry Analysis. XLV Mexican Conference on Biomedical Engineering. CNIB 2022. IFMBE Proceedings, vol 86. Springer, Cham. doi: org/10.1007/978-3-031-18256-3_20
- Zaidi, S. T. R.; Weier, N. E. Bacterial Infections and the Role of the Pharmacist, Editor(s): Zaheer-Ud-Din Babar. Encyclopedia of Pharmacy Practice and Clinical Pharmacy, Elsevier. 2019, 730-741, ISBN 9780128127360, doi: org/10.1016/B978-0-12-812735-3.00556-2.
- Sinthupoom, N.; Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul,V. Eur. Food. Res. Technol. 2015, 240, 1–17. https://doi.org/10.1007/s00217-014-2354-1
- Cavassin, F. B.; Baú-Carneiro, J. L.; Vilas-Boas, R. R.; Queiroz-Telles, F. Infect. Dis. Ther. 2021, 10, 115–147. doi: 10.1007/s40121-020-00382-7.
- Xiao, Y.; Yuan, P.; Sun, Y.; Xu, Y.; Deng, X.; Wang, X.; Liu, R.; Chen, Q.; Jiang, L. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 133, 282–291. doi: 10.1016/j.oooo.2021.10.023.
- Emami, L.; Faghih, Z.; Ataollahi, E.; Sadeghian, S.; Rezaei, Z.; Khabnadideh, S. Curr. Med. Chem. 2023, 30(2), 220-249. doi: 10.2174/0929867329666220407094430.
- Liu, C. G.; Green, S. I.; Min, L.; Clark J. R.; Salazar, K. C.; Terwilliger, A. L.; Kaplan, H. B.; Trautner, B. W.; Ramig, R. F.; Maressoa, A. W. mBio® 2020, 11(4), e01462-20. doi: 10.1128/mBio.01462-20.
- a) Palmer, J. D.; Foster, K. R. Proc. Natl. Acad. Sci. USA. 2022, 119(38), e2205407119-30. doi: 10.1073/pnas.2205407119.
- Kapoor, G.; Saigal, S.; Elongavan, J. Anaesthesiol. Clin. Pharmacol. 2017, 33(3), 300-305. doi: 10.4103/joacp.JOACP_349_15.
- Nemeth, J.; Oesch, G.; Kuster S. P. J. Antimicrob. Chemother. 2015, 70(2), 382-395. doi: 10.1093/jac/dku379.
- Pancu, D. F.; Scurtu, A.; Macasoi, I. G.; Marti, D.; Mioc, M.; Soica, C.; Coricovac, D.; Horhat, D.; Poenaru, M.; Dehelean, C. Antibiotics. 2021, 10(4), 401-436. doi:org/10.3390/antibiotics10040401
- Mitcheltree, M. J.; Pisipati, A.; Syroegin, E. A.; Silvestre, K. J.; Klepacki, D.; Mason, J. D.; Terwilliger, D. W.; Testolin, G.; Pote, A. R.; Y. K. J.; Wu, Ladley, R. P.; Chatman, K.; Mankin, A. S.; Polikanov, Y. S.; Myers, A. G. Nature. 2021, 599, 507–512. doi: org/10.1038/s41586-021-04045-6
- Martyn, J. A.; Paliadelis, P.; Perry, C. Nurse Educ. Pract. 2019, 37, 109-114. doi: 10.1016/j.nepr.2019.05.006
- a) Samrot, A. V.; Wilson, S.; Preeth, R. S. S.; Prakash, P.; Sathiyasree, M.; Saigeetha, S.; Shobana, N.; Pachiyappan, S.; Rajesh, V. V. Sustainability 2023, 15, 12639-12663. https://doi.org/10.3390/su151612639 b) Danner, M-C.; Robertson, A.; Behrends, V.; Reiss, J. Science of The Total Environment 2019, 664, 793-804. https://doi.org/10.1016/j.scitotenv.2019.01.406
- Hanna, N.; Sun, P.; Sun, Q.; Li, X.; Yang, X.; Ji, X.; Zoub, H.; Ottosond, J.; Nilssone, L. E.; Berglunde, B.; Dyara, O. J.; Tamhankara, A. J.; Lundborg, C. S. Environ Int. 2018, 114, 131–142.
- Shi, H.; Ni, J.; Zheng, T.; Wang X.; Wu, C.; Wang, Q. Environ. Chem. Lett. 2020, 18, 345–360. https://doi.org/10.1007/s10311-019-00945-2
- Su, Z., Chen, L.; Front. Environ. Sci. Eng. 2024, 18, 36-46. https://doi.org/10.1007/s11783-024-1796-3
- Akhil, D.; Lakshmi, D.; Kumar, P. S.; Vo, D-V. N.; Kartik, A. Environ. Chem. Lett. 2021, 19, 1477–1507 . https://doi.org/10.1007/s10311-020-01152-0
- Sodhi, K. K.; Kumar, M., Balan, B.; Singh Dhaulaniya, A.; Shree, P.; Sharma, N.; Singh, D. K. SN Appl. Sci. 2021, 3, 269-293. https://doi.org/10.1007/s42452-020-04003-3
- Tian, M.; He, X.; Feng, Y.; Wang, W.; Chen, H.; Gong, M.; Liu, D.; Clarke, J. L.; van Eerde, A. Antibiotics 2021, 10, 539-554. https://doi.org/10.3390/antibiotics10050539
- Mohsen, S.; Dickinson, J. A.; Somayaji, R. Can. Fam. Physician. 2020, 66(9), 651-659.
- Yang, Q.; Gao, Y.; Ke, J.; Show, P. L.; Ge, Y.; Guo, R.; Chen, J. Bioengineered 2021, 12(1), 7376-7416. doi: 10.1080/21655979.2021.1974657
- Yalew, S. T. Biomed. J. Sci. & Tech. Res. 2020, 24(5), 18651-18658.
- Egorov, M.; Ulyashova, M. M.; Rubtsova, M. Y. Acta Naturae. 2018, 10(4), 33-48, doi:10.32607/20758251-2018-10-4-33-48
- a) Chin, K. W.; Tiong, H. L. M.; Luang-In, V.; Ma, N. L. Environmental Advances. 2023, 11, 100331-100341. doi: org/10.1016/j.envadv.2022.100331 b) Nwobodo, C. D.; Ugwu, M. C.; Anie, O. C.; Al-Ouqaili, M. T. S.; Ikem, C. J.; Chigozie, V. U.; Saki, M. J. Clin. Lab. Anal. 2022, 36(9), e24655- e24665. doi: 10.1002/jcla.24655. c) Sun, G.; Zhang, Q.; Dong, Z.; Dong, D.; Fang, H.; Wang, C.; Dong, Y.; Wu, J.; Tan, X.; Zhu, P.; Wan, Y. Front. Public Health 2022, 10, 1002015-1002039. doi: 10.3389/fpubh.2022.1002015. d) Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotics (Basel) 2022, 11(8), 1079-1119. doi: 10.3390/antibiotics11081079. e) Begum, S.; Begum, T.; Rahman, N.; Khan, R. GSC Biological and Pharmaceutical Sciences. 2021, 14(02), 087-097. doi:org/10.30574/gscbps.2021.14.2.0037
- Giacomini, E.; Perrone, V.; Alessandrini, D.; Paoli, D.; Nappi, C.; Degli, E. L. Infect Drug Resist. 2021, 14, 849-858. doi: 10.2147/IDR.S289741.
- El-Saber Batiha, G.; Hussein, D. E.; Algammal, A. M.; George, T. T.; Jeandet, P.; Al-Snafi, A. E.; Tiwari, A.; Pagnossa, J. P.; Lima, C. M.; Thorat, N. D.; Zahoor, M.; El-Esawi, M.; Dey, A.; Alghamdi, S.; Hetta, H. F.; Cruz-Martins, N. Food Control 2021, 126, 108066-108080. doi: org/10.1016/j.foodcont.2021.108066.
- Kirchhelle, C. Palgrave Commun. 2018, 4(1), 96-100. doi: org/10.1057/s41599-018-0152-2
- Ramirez, J.; Guarner, F.; Bustos, F. L.; Maruy, A.; Sdepanian, V. L.; Cohen H. Front. Cell Infect. Microbiol. 2020, 10, 572912-572922. doi:10.3389/fcimb.2020.572912.
- Nori, P.; Cowman, K.; Chen, V.; Bartash, R.; Szymczak, W.; Madaline, T.; Punjabi, K. C.; Jain, R.; Aldrich, M.; Weston, G.; Gialanella, P.; Corpuz, M.; Gendlina, I.; Guo, Y. Infect. Control Hosp. Epidemiol. 2021, 42(1), 84-88. doi: 10.1017/ice.2020.368.
- Kubeček, O.; Paterová, P.; Novosadová, M. Life (Basel) 2021, 11(12), 1387-1411. doi: 10.3390/life11121387.
- Keitoku, K.; Nishimura, Y.; Hagiya, H.; Koyama, T.; Otsuka, F. Int. J. Infect. Dis. 2021, 111, 12-20. doi: 10.1016/j.ijid.2021.08.018.
- Musoke, D.; Namata, C.; Lubega, G. B.; Niyongabo, F.; Gonza, J.; Chidziwisano, K.; Nalinya, S.; Nuwematsiko, R.; Morse, T. Environ. Health Prev. Med. 2021, 26, 100-105. https://doi.org/10.1186/s12199-021-01023-2
- Limato, R.; Lazarus, G.; Dernison, P.; Mudia, M.; Alamanda, M.; Nelwan, E. J.; Sinto, R.; Karuniawati, A.; Doorn, H. R. v.; Hamers, R. L. The Lancet Regional Health – Southeast Asia 2022, 2, 100013-35. doi: org/10.1016/j.lansea.2022.05.002.
- Sharland, M.; Cappello, B.; Ombajo, L. A.; Bazira, J.; Chitatanga, R.; Chuki, P.; Gandra, S.; Harbarth, S.; Loeb, M.; Mendelson, M.; Moja, L.; Pulcini, C.; Tacconelli, E.; Zanichelli, V.; Zeng, M.; Huttner, B. D. Lancet. Infect. Dis. 2022, 22(11), 1528-1530. doi: 10.1016/S1473-3099(22)00683-1.
- Årdal, C.; Balasegaram, M.; Laxminarayan, R.; McAdams, D.; Outterson, K.; Rex, J. H.; Sumpradit N. Nat. Rev. Microbiol. 2020, 18, 267–274. https://doi.org/10.1038/s41579-019-0293-3
- Bloomer, E.; McKee, J. Public Health Pol. 2018, 39, 389–406. https://doi.org/10.1057/s41271-018-0144-x
- Zhao, F.; Yang, L.; Yen, H.; Feng, Q.; Li, M.; Chen, L. Nat. Commun. 2023, 14, 6094-6104. https://doi.org/10.1038/s41467-023-41258-x
- Reverter, M.; Sarter, S.; Caruso, D.; Avarre, J. C.; Combe, M.; Pepey, E.; Pouyaud, L.; Vega-Heredía, S.; de Verdal, H.; Gozlan, R. E. Nat. Commun. 2020, 11(1), 1870-1878. doi:10.1038/s41467-020-15735-6.
- Gupta, A.; Vyas, R. K.; Vyas, S. Separation Science and Technology 2023, 58(2), 326-344. doi: 10.1080/01496395.2022.2110120
- Yang, L.; Lyu, J.; Zhang, L.; Wang, L.; Yu, J.; Cao, Z.; Tudi, M.; Meng, M. Environ. Sci. Pollut. Res. 2023, 30, 112863–112876. https://doi.org/10.1007/s11356-023-30087-6
- Kosiyaporn, H.; Chanvatik, S.; Issaramalai, T.; Kaewkhankhaeng, W.; Kulthanmanusorn, A.; Saengruang, N.; Witthayapipopsakul, W.; Viriyathorn, S.; Kirivan, S.; Kunpeuk, W.; Suphanchaimat, R.; Lekagul, A.; Tangcharoensathien, V. PLoS One 2020, 15(1), e0227973-999. doi: 10.1371/journal.pone.0227973.
- Pancu, D. F.; Scurtu, A.; Macasoi, I. G.; Marti, D.; Mioc, M.; Soica, C.; Coricovac, D.; Horhat, D.; Poenaru, M.; Dehelean, C. Antibiotics (Basel) 2021, 10(4), 401-436. doi: 10.3390/antibiotics10040401.
- Li, Q.; Pellegrino, J.; Lee, D. J.; Tran, A. A.; Chaires, H. A.; Wang, R.; Park, J. E.; Ji, K.; Chow, D.; Zhang, N.; Brilot, A. F.; Biel, J.T.; van Zundert, G.; Borrelli, K.; Shinabarger, D.; Wolfe, C.; Murray, B.; Jacobson, M. P.; Mühle, E.; Chesneau, O.; Fraser, J. S.; Seiple, I. B. Nature 2020, 586(7827), 145-150. doi: 10.1038/s41586-020-2761-3.
- Grenni, P.; Ancona, V.; Caracciolo, A. B. Microchemical Journal 2018, 136, 25-39, doi: org/10.1016/j.microc.2017.02.006.
- Sharda, N.; Kumar, D.; Thakur, R.; Sharma, A. K.; Sankhyan, S.; Kumar, A. Water Air & Soil Pollut. 2023, 234(11), 683. https://doi.org/10.1007/s11270-023-06695-w
- Deb Adhikari, M.; Saha, T.; Tiwary, B. K. (2022). Quest for Alternatives to Antibiotics: An Urgent Need of the Twenty-First Century. In: Saha, T.; Deb Adhikari, M.; Tiwary, B. K. (eds) Alternatives to Antibiotics. Springer, Singapore. https://doi.org/10.1007/978-981-19-1854-4_1
- Osman, A-H.; Kotey, F. C. N.; Odoom, A.; Darkwah, S.; Yeboah, R. K.; Dayie, N. T. K. D.; Donkor, E. S. Antibiotics 2023, 12, 1329. https://doi.org/10.3390/antibiotics12081329
- Kaul, G.; Shukla, M.; Dasgupta, A.; Chopra, S. (2019). Alternative Therapies to Antibiotics to Combat Drug-Resistant Bacterial Pathogens. In: Ahmad, I., Ahmad, S., Rumbaugh, K. (eds) Antibacterial Drug Discovery to Combat MDR. Springer, Singapore. https://doi.org/10.1007/978-981-13-9871-1_9
- Xu, Q.; Hu, X.; Wang, Y. Mol. Biotechnol. 2021, 63, 1103–1124. https://doi.org/10.1007/s12033-021-00371-2
- a) Urnukhsaikhan, E.; Bold, B. E.; Gunbileg, A.; Sukhbaatar, N.; Mishig‑Ochir T. Sci. Rep. 2021, 11, 21047-21058. https://doi.org/10.1038/s41598-021-00520-2 b) Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Int. J. Mol. Sci. 2021, 22(13), 7202-7222. doi:10.3390/ijms22137202
- Li, S.; Jiang, S.; Jia, W.; Guo, T.; Wang, F.; Li, J.; Yao, Z. Food Chem. 2024, 432, 137231-137241. doi: 10.1016/j.foodchem.2023.137231.

This work is licensed under a Creative Commons Attribution 4.0 International License.










