Novel Tricyclic Functionalized Azetidinone Molecules: Synthesis, Characterization and Evaluation of Antidepressant Action
Lakshmi Narain College of Pharmacy, Bhopal, M. P, India.
Corresponding Author E-mail: lncpbho@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400225
Article Received on : 24 Feb 2024
Article Accepted on :
Article Published : 17 Apr 2024
Reviewed by: Dr. Shaikh Azam
Second Review by: Dr. Jagadeesh Kumar
Final Approval by: Dr. Murat HATİPOĞLU
In order to assess the depression decreasing effect of the molecules, a forced swim protocol in rats was used to manufacture azetidinone compounds with a tricyclic nucleus. Three different procedures were used in order to synthesize the necessary compounds. Step one involved reacting anthracene-9,10-dione with hydrazine hydrate in the presence of methanol under refluxing conditions to produce 2. Next, several substituted aromatic aldehydes were refluxed with 2 under the influence glacial acetic acid as catalyst to create Schiff bases. Chloracetyl chloride was used under alkaline circumstances to accomplish cyclization in the last stage. The produced compounds 4a-e had a yield range from 61-70% and shown solubility or mild solubility in DMSO, methanol, and chloroform. Using methanol:ethyl acetate (4:6) as the solvent solution, TLC was used to evaluate the purity of the produced compounds. The compounds' Rf values ranged from 0.59 to 0.73. The compounds have a melting temperature range of 172-214°C and were produced as a white to yellow solid. The compounds demonstrated antidepressant activity that was dosage dependent. Compounds 4a, 4b, and 4d had significantly shorter immobility times than the control group. It became clear that each test chemical may exhibit antidepressant activity when the dosage was increased.
KEYWORDS:Azetidinone; Depression; Forced swim test; Spectral Characterization;; Synthesis
Download this article as:| Copy the following to cite this article: Bhadre D, Parulben D. M. Novel Tricyclic Functionalized Azetidinone Molecules: Synthesis, Characterization and Evaluation of Antidepressant Action. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Bhadre D, Parulben D. M. Novel Tricyclic Functionalized Azetidinone Molecules: Synthesis, Characterization and Evaluation of Antidepressant Action. Orient J Chem 2024;40(2). Available from: https://bit.ly/43ZaCF3 |
Introduction
Among the most prevalent mental illnesses is depression. It is a diverse condition that has been described and categorized in a number of ways 1. Mood changes (mania or depression) rather than disruptions in cognition are the main hallmarks of affective disorders. The “monoamine hypothesis,” which holds that depression results from a functional deficiency of monoamine transmetters at specific brain locations, is the primary biochemical explanation explaining depression. Every antidepressant on the market today works by selectively antagonistic receptors, reabsorbing serotonin, norepinephrine, or both.
A prevalent mental health condition that impacts a significant number of people is depression, according to epidemiological data. There are 350 million individuals believed to be afflicted by depression globally, according to the World Health Organization (WHO). Typical mood swings and fleeting emotional reactions to problems encountered in daily life are not the same as depression 2.
These days, the oftenly prescribed medications as antidepressant, like tricycylics are restricted because of the potential side effects they carry, which include anorexia, cardiac failure, diarrhea, loss in concentrating, giddiness, sleepiness, dysrhythmias, gastric upset, extreme central stimulation (excitation, seizure), reduced blood pressure, sleeplessness, liver impairment, obsession, biliousness, palpitations, quiver, feebleness and lassitude, and many more 3-5.
By taking use of the strain energy connected to it, the four-membered cyclic lactam (ß-lactam) skeleton known as azetidin-2-one has been identified as an appealing target of modern chemical synthesis of a multitude of organic compounds 6. Many therapeutic effects, such as those that are antimycobacterial, depressive, antibacterial, and more, have been linked to the nucleus.
The use of a simple nitrogen-bearing heterocycle, such as azetidinone, as a component of a larger molecule may be able to present antidepressant action and may also inculcate properties favourable for interaction with the enzymes involved in depression 7. This theory is based on the aforementioned limitations and the growing demand for antidepressant drugs.
Material and Methods
The scheme for synthesis of azetidinone was adapted from report by Malviya et al8 and Kerzare et al 9. The methodology was modified according to laboratory conditions in order to obtain product in high yields.
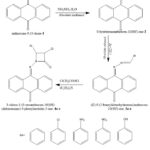 |
Scheme 1: Synthetic route for azetidin-2-one derivatives. |
Synthesis of 9-hydrazonoanthracen-10(9H)-one, 2
Anthracene-9,10-dione (20.82 g, 0.1 mol) was weighed and combined with hydrazine hydrate (99%, 5.05 g, 1.1 mol) in 100 milliliters of absolute methanol. The combination was allowed to react under reflux for 1 hour and then let come down to room conditions. The hydrazone pale yellow crystals that precipitated out have been filtered using glass wool and dried. To get pure hydrazine, ethanol was used to recrystallize the crude product 8.
General method for synthesis of 9-(2-benzylidenehydrazono)anthracen-10(9H)-one, 3a-e
A solution of compound 2 (0.01 mol) in 60 mL ethanol has been mixed with a few drops of glacial acetic acid and desired benzaldehyde (0.01 mol). After that, the mixture was refluxed for eight hours. The leftover liquid was cooled to attain room temperature and then poured over crushed ice, and filtered. The surplus ethanol was allowed to evaporate. Using 70% ethanol, the crude product (3a-e) was obtained and recrystallized 8.
Synthesis of azetidin-2-one derivatives, 4a-e
1,5 mL of 1, 4-Dioxane was used to dissolve a combination of triethylamine (0.02 mol) and Schiff base (3a-e) (0.01 mol). After that, quantities of a chloroacetyl chloride solution (0.02 mol) had been added followed by vigorous shaking at laboratory ambient conditions for 20 minutes. The reaction content was heated under reflux for three hours, and the contents were settled down to room temperature for forty-eight hours before being transferred into ice-cold water. To achieve pure 4a-e, the resultant solid had been filtered, repeatedly washed using water, and then recrystallization was achieved from 70% ethanol 9.
3-chloro-1-(9-oxoanthracen-10(9H)-ylideneamino)-4-phenylazetidin-2-one, 4a
Pale Yellow in Color; 386.08 (m/e, calculated); NMR: 8.20–6.69 CH-Benzene, 1.25-2.66 CH-aliphatic; FT-IR (cm-1): 2935.24 (C–C stretching (cyclic)), 3230.00 (C–H stretching), 1704.43 (C=O stretching), 1650.77, 1611.08 (C–C stretching (Aromatic)), 1526.08 (C=N stretching), 1380.80 (C-N stretching), 1095.29 (C-Cl stretching).
3-chloro-4-(4-chlorophenyl)-1-((10-oxoanthracen-9(10H)-ylidene) amino) azetidin-2-one, 4b
FT-IR (cm-1): 2982.69 (C-C stretching (cyclic)), 3230.63 (C-H stretching), 1710.04 (C=O stretching), 1646.30 (C-C stretching (Aromatic)), 1519.35 (C=N stretching), 1349.64 (C-N stretching), and 1090.04 (C-Cl stretching); Color: Dark Yellow; Mass (m/e, calculated): 421.28; 1H-NMR (δ ppm): 7.26-7.99 (CH-Benzene), 1.25-2.66 (CH-aliphatic);
4-(4-aminophenyl)-3-chloro-1-((10-oxoanthracen-9(10H)-ylidene) amino) azetidin-2-one, 4c
Color: white; Calculated mass (m/e): 401.85. 1H-NMR (δ ppm): 6.72–7.97 (CH-Benzene), 2.2 (CH-aliphatic), and 3.9 (NH-Amine); FT-IR (cm-1): 2992.19 (C-C stretching (cyclic)), 3099.37 (C-H stretching), 1726.28 (C=O stretching), 1687.76, 1613.90 (C-C stretching (Aromatic)), 1527.62 (C=N Stretching), 1377.97 (C-N stretching), and 1093.52 (C-Cl stretching).
3-chloro-4-(4-nitrophenyl)-1-((10-oxoanthracen-9(10H)-ylidene)amino) azetidin-2-one, 4d
Color: White; Mass (m/e, calculated): 431.83, 1H-NMR (δ ppm): 6.95-7.99 (CH-Benzene), 3.0 (CH-aliphatic); FT-IR (cm-1): 2836.96 (C-C stretching (cyclic)), 1914.84 (C=O stretching, Fermi), 1610.30 (C-C stretching (Aromatic)), 1575.66 (C=N Stretching), 1379.66 (C-N stretching), 1046.29 (C-Cl stretching), 1462.57 (NO2 stretching)
3-chloro-4-(4-hydroxyphenyl)-1-((10-oxoanthracen-9(10H)-ylidene)amino) azetidin-2-one, 4e
Color: Yellow; Mass (m/e, calculated): 402.83, 1H-NMR (δ ppm): 7.0-8.2 (CH-Benzene), 1.8-2.3 (CH-aliphatic), 4.2 (OH-Phenolic); FT-IR (cm-1): 2933.32 (C-C stretching (cyclic)), 3232.34 (C-H stretching), 1703.85 (C=O stretching), 1651.13, 1613.97 (C-C stretching (Aromatic)), 1514.07 (C=N Stretching), 1392.25 (C-N stretching), 1082.53 (C-Cl stretching), 3732.89 (O-H stretching)
The synthesized compounds were subjected to characterization for retention factor by thin layer chromatography, solubility, melting point, yield, 1H NMR, and IR spectroscopy 10-12.
Antidepressant Action
Animal
The study included male or female albino mice weighing between 25 and 30 g. Grouped together, the animals were kept at the institute’s animal home in 38x23x10 cm polyacrylic cages. Within each cage, no more than four animals were kept in standard conditions in a natural light and dark sequence (14 hours light and 10 hours dark, 27±2°C, and 44-56% relative humidity). The animals were allowed unlimited access to Golden Feeds, India’s standard diet, as well as tap water, for one week to help them get used to their surroundings before and during the experiments.
Acute toxicity study
Each test substance was given orally to three animals in total, at a dosage of 2000 mg/kg. The animals were monitored separately for the first thirty minutes following dosage, then every day for the next fourteen days. After that, they were observed on a sporadic basis for the first twenty-four hours. Every day, changes were noted in the skin, fur, eyes, and mucous (nasal), alongside change in the respiration, circulation (cardiac rate and arterial press), autonomic nervous system (saliva secretion, sweat, urine incontinence, and defecation), and central nervous system (sleepiness, quivers, and seizures). Throughout the course of two weeks, any deaths that occurred were also noted 13.
Experimental Setup
In order to perform the study, the animal had been separated as seven groups, each containing six individuals. Group VII functioned as the positive control and received fluoxetine (10 mg/kg, i.p). Group I was given normal saline as the control, while groups II-VI were administered of the test compounds (50 mg/kg, i.p.) 14.
Forced Swim Test
A standard volume of 0.05 mL per 20 g body weight was administered intraperitoneally into each mouse 30 minutes before the test, using the dissolved synthetic chemicals and fluoxetine. To measure the effect of the test material, mice were placed one by one in a glass cylinder of 25 cm in height and 10 cm in diameter. The water was kept at a temperature between 22 and 25°C up to a height of 10 cm. Throughout the course of the test, each mouse was given six minutes to swim, and during the last four minutes, the length of immobility was recorded and observed. The mouse was considered to be immobile throughout the period during which it was floating in the water, not fighting, and moving barely enough to maintain its head above the surface 15.
Results
Chemistry
The retention factor (Rf), solubility, yield (%), and melting point (°C) of the five synthesized azetidinone derivatives were noted in the laboratory and classified as 4a–e.
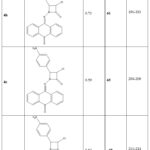 |
Table 1: Yield, Melting point and Rf value of synthesized molecules. |
All the compounds were insoluble in water and soluble or partially soluble in methanol, chloroform and dimethylsulfoxide (DMSO).
The thin layer chromatography of all the synthesized derivatives was carried out using precoated silica gel G TLC plates, using methanol: ethyl acetate (4:6) as the solvent system and the retention factor (Rf) value was calculated (Table 1).
Using the open capillary technique and the melting point equipment, the melting points of the compounds 4a–e were found. (Table 1).
Antidepressant action
The antidepressant action of the synthesized azetidin-2-ones was assessed using forced swim test in mice.
Acute toxicity
The produced molecules were given orally to the test animal at a dosage of 2000 mg/kg in order to conduct the acute toxicity test. Since no animal perished, the dosage of up to 2000 mg/kg was deemed safe. Since no animal perished, the LD50 was determined to be greater than 2000 mg/kg, and any dosage below 2000 mg/kg would be taken into consideration for assessing the biological activities. For the trial, dosages as low as 40 mg/kg and 60 mg/kg bodyweight were chosen.
Forced Swim Test
The test animal were administered the synthesized molecules at 40 & 60 mg/Kg dose and the immobility time was recorded as the indicator of antidepressant effect (Table 2).
Table 2: Immobility time in FST.
|
Treatment |
Immobility time (sec, mean ± SD, n=6) |
|||
|
Dose (mg/kg) |
||||
|
– |
10 |
40 |
60 |
|
|
Control |
29.17 ± 5.0760 |
– |
– |
– |
|
Fluoxetine |
– |
9.5 ± 1.2247 |
– |
– |
|
4a |
– |
– |
22.16 ± 3.9707 |
12.16 ± 2.2860 |
|
4b |
– |
– |
23.67 ± 1.6329 |
22.17 ± 3.9707 |
|
4c |
– |
– |
26.67 ± 2.9439 |
23.67 ± 1.9663 |
|
4d |
– |
– |
20.17 ± 3.0605 |
17.5 ± 2.4289 |
|
4e |
– |
– |
25.67 ± 2.2509 |
23.67 ± 1.6329 |
The statistical analysis of the mouse immobility data was conducted using GraphPad Prism software, employing one-way ANOVA and Dunnett’s test to assess the antidepressant activity of the examined compound
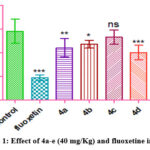 |
Figure 1: Effect of 4a-e (40 mg/Kg) and fluoxetine in FST. |
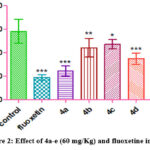 |
Figure 2: Effect of 4a-e (60 mg/Kg) and fluoxetine in FST |
Discussion
Chemistry
Over the years, the synthesis of 2-azetidinones has drawn a lot of interest, and many techniques are known to be used in their manufacture 16-19. In order to synthesize the desired molecules, three separate steps had to be completed: the first involved reacting anthracene-9,10-dione with hydrazine hydrate in the presence of methanol under reflux conditions, yielding 2; the second involved refluxing 2 with different benzaldehydes under the influence of glacial acetic acid as catalyst, forming Schiff bases; and the third and final step involved cyclization using chloracetyl chloride in an alkaline environment. These items met the requirements for yield and purity based on melting point and thin layer chromatography examinations.
IR and 1HNMR spectroscopy were used to determine the compounds’ structures.Twenty The stretching vibration peaks at 1400–1200 cm-1, 1760–1700 cm-1, 1600–1450 cm-1, and 1100–1020 cm-1 (middle) were seen in the IR spectra of every compound. These peaks were caused by C–N, C=O, C=N, and C–Cl. Other vibrations from aromatic C-C cyclic, aromatic C-C, and aromatic C-H were also visible in the spectra.
The peaks of aromatic and aliphatic CH were seen in the acquired 1HNMR spectra, respectively, at 6.7–7.2 and 2-3.3 ppm.
Antidepressant action
Owing to the clinical potential displayed by various tricyclic antidepressant molecules, the antidepressant effects of the synthesized molecules 4a-e were evaluated by FST. The chemicals’ dose-dependent antidepressant activity was evident from the data. According to Figure 5.1, there was a statistically significant (p<0.01) decrease in the immobility time for compounds 4a, 4b, and 4d when compared to the control group. However the results obtained by compounds 4c and 4e were not significant at the lower dose, signifying that increasing polarity diminishes the chances of the molecules to enter the central nervous system.
On increasing the dose of test compounds, it was evident that all the test compounds were able to show antidepressant action (Figure 2).
FST is a very frequently used behavioral distress model employing rodent to predict the antidepressant potential of drugs and molecules. The model is based on the concept that mice when forced to swim initially try to perform the escape behavior activity and when they experience helplessness they exhibit the immobile posture. The immobility behavior has been accepted to be associated to depression and the reduction in the immobility is used as a measure of antidepressant effect 20,21.
Conclusion
Among mental illnesses, depression is among the most prevalent. Imidamazine, desipramine, and fluoxetine are the three medications for depression that are most often administered. A simple nitrogen-bearing heterocycle, such as azetidinone, may be used to present antidepressant action and may also instill properties favorable for interaction with the enzymes involved in depression. This theory is based on the numerous limitations and growing demand for antidepressant drugs. The objective of this work was to synthesize azetidinone compounds containing tricyclic nucleus and evaluate the antidepressant action of the molecules using forced swim test in rodent. It could be concluded from the study, that azetidin-2-ones containing tricyclic planar structure present a novel approach to design and synthesis of new antidepressant molecules.
Acknowledgement
Thanks are due to RB Science, Bhopal for providing the facilities for the synthetic studies.
Conflict of Interests
The authors declare no conflict of interests.
Funding Sources
References
- Srinath, R.; Pinkal, D. V.; Saravanan, J.; Pravin, S. J.; Prashant, R.; Aravind, S. Int. J. Res. Pharm. Sci. 2010, 1, 143–50.
- Lucas, M. C.; Weikert, R. J.; Carter, D. S.; Cai, H. Y.; Greenhouse, R.; Lyer, P. S. Bioorg. Med. Chem. Lett. 2010, 20, 5559–66.
CrossRef - O’Donnell, J. M.; Bies, R. R.; Shelton, R. C. In: Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 13 ed. 2017, pp 267-279
- Sabella, D. Am. J. Nursing. 2018, 118 (9), 52-89
CrossRef - Rey, J. A. In: Lippincott Illustrated Reviews: Pharmacology. 2015, pp 135-146
- Anusha, K.; Pradeep Kumar, V.; Vara Prasad, M.; Bakta Markandeya Raju, V.; Gopinath, C. J. Global Trends Pharm. Sci. 2015, 6(1), 2388-2402
- Kusurkar, R. V.; Rayani, R. H.; Parmar, D. R.; Bhoi, M. N.; Zunjar, V. H.; Soni, J. Y. Results Chem. 2022, 4: 100357. Doi: 10.1016/j.rechem.2022.100357
CrossRef - Malviya, M.; Mishra, B.; Korde, B. J. Pharmacol, Biomed. 2021, 5(2), 286-294
- Kerzare, D. J.; Menghani, S. S.; Khedekar, P. B. Indian J. Pharm. Edu. Res. 2018, 52(1), 110-121
CrossRef - Ault, A. In: Techniques and experiments for organic chemistry. 1998, pp. 138-240.
- Ault, A. In: Techniques and experiments for organic chemistry. 1998, pp. 44-37.
- Thin layer chromatography. Available from https://lab-training.com/thin-layer-chromatography-tlc/; assessed on 10/08/2023
- OECD guidleines for toxicity testing. Available from https://ntp.niehs.nih.gov/sites/default/files/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf
- Thomas, A. B.; Nanda, R. K.; Kothapalli, L. P.; Hamane, S. C. Arabian J. Chem. 2016, 9(S1), S79-S90
CrossRef - Mathur, Y.; Jain, A. J. Pharmacol. Biomed. 2021, 5(4), 360-365
- Xu, J. Mol. Divers. 2005, 9, 45–49. https://doi.org/10.1007/s11030-005-1306-x
CrossRef - Zarei, M. Monatsh Chem. 2013, 144, 1021–1025. https://doi.org/10.1007/s00706-012-0918-y
CrossRef - Cebeci, Y. U.; Bayrak, H.; Şirin, Y. Bioorg. Chem. 2019; 88, 102928. https://doi.org/10.1016/j.bioorg.2019.102928
CrossRef - Fifield, F. W.; Kealy, D. Principles and Practice of Analytical Chemistry. 5th ed, 2000.
- Oliveira, C. E.; Sari, M. H.; Zborowski, V. A.; Araujo, P. C.; Nogueira, C. W.; Zeni, G. Pharmacol. Biochem. Behav. 2017, 154, 31–38.
CrossRef - Cryan, J. F.; Mombereau, C.; Vassout, A. Neurosci Behav Rev. 2005, 29, 571–625.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










