Influence of Shear Speed on the Rheological Behavior of Chocolate
University of Bucharest, Faculty of Chemistry, Department of Physical Chemistry, 4-12 Elisabeta Blvd, 030018, Bucharest, Romania.
Corresponding Author E-mail: istanciu75@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/400215
Article Received on : 28 Feb 2024
Article Accepted on :
Article Published : 24 Apr 2024
Reviewed by: TIRSOAGA-JURCA Alina
Second Review by: Dr. Noureddine Ouerfelli
Final Approval by: Dr. Tanay Pramanik
This article proposes a polynomial equation and another exponential equation of the second order that describes the rheological behavior of chocolate. The second-order polynomial and exponential functions describe the non-Newtonian behavior of chocolate in the temperature range of 36 and 44°C. The rheological study of the chocolate was carried out at the shear speed of the chocolate wa between 0.1s-1 and 68s-1.
KEYWORDS:Chocolate; Equation; Rheology; Temperature
Download this article as:| Copy the following to cite this article: Stanciu I. Influence of Shear Speed on the Rheological Behavior of Chocolate. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Stanciu I. Influence of Shear Speed on the Rheological Behavior of Chocolate. Orient J Chem 2024;40(2). Available from: https://bit.ly/3JvBYJB |
Introduction
Chocolate was first manufactured in Italy by a confectioner from Florence who noticed that cocoa powder dissolves in water or milk. He then added vanilla and sugar.
Chocolate has the properties of thixotropy and when heated they become fluid. The dispersed phase is represented by the solid particles of cocoa and powdered sugar and the dispersion phase is represented by the cocoa melt.
According to the composition, chocolate is classified into:
simple: regular, sweet, without sugar, blanket or granulated;
in a homogeneous mixture: regular with milk, blanket with milk, granules with milk.
According to the way of forming the covers for chocolates with filling, they can be:
chocolate specialties manufactured by casting: tablets, fine chocolate candies;
chocolate specialties manufactured by coating: extra fine chocolate candies;
chocolate specialties by mixing: with expanded cereals.
According to the composition of the fillings, they can be:
cream fillings: simple fondant, chocolate, fatty kernels, with sugar, jellies, candied or preserved fruits;
liquid fillings: alcohol syrup, alcoholic beverages, fruit juices;
foam fillings;
nougat or caramel fillings;
waffle or cocoa fillings 1-5.
According to the composition and the processing process, the following types of chocolate are manufactured:
chocolate without additives or natural. It contains cocoa mass, cocoa butter, sugar and aromatic substances. This type of chocolate is subdivided into:
Regular chocolate;
Dessert chocolate that contains a lower amount of sugar and a higher amount of cocoa mass and cocoa butter than regular chocolate. The cocoa is first refined in rollers, and after that the refining continues through a conching process;
Chocolate dust contains a slightly higher amount of sugar than ordinary chocolate, without the addition of cocoa butter, so that it can be obtained in powder form. Chocolate powder differs from cocoa powder in that no sugar should be added when using it, as this is provided for in the recipe.
chocolate with additions is subdivided into:
Milk chocolate – contains milk, which is usually introduced in the form of milk powder; b) Chocolate mignon with the addition of roasted and rubbed almonds, apricot or peach kernels;
Chocolate with nuts – contains whole or chopped nuts (barot), roasted, especially hazelnuts, evenly dispersed in the chocolate mass;
Chocolate with coffee – contains ground coffee or coffee extract;
Chocolate with wafers – contains firmites with wafers, evenly scattered in the chocolate mass;
Fruit chocolate – contains candied, finely chopped fruit, dried or smooth fruit.
According to shape and size, they are distinguished:
chocolate in rectangular tablets, weighing 100 g and smaller (minimum 12 g). It is the most common form of chocolate;
chocolate in small rectangular or round tablets – maximum 10 g;
chocolate in figures – in the form of full or empty figures; bombs, animals, cedar cones;
chocolate with drawings – flat figures, in relief, of small size 6-8.
For the manufacture of products covered with chocolate (fondants, wafers) a semi-finished chocolate-covering is usually used. It contains a lot of fat (minimum 33%) to form a fluid mass, with which the products are easily glazed.
A porous chocolate called “slava” is also made. This product has a spongy structure, with many pores having a diameter of 0.5-3 mm. The taste of porous chocolate is smoother than regular chocolate. Porous chocolate is obtained by beating the liquid chocolate mass, pouring it into molds and forming a vacuum. After the formation of the porous mass of chocolate (due to the expansion of air bubbles in a vacuum), it cools quickly in the same room until it solidifies 9-12.
In this article, we found by fitting the polynomial and exponential order of two the rheological equations of chocolate at temperatures between 36 and 44 °C and shear rates between 0.1 and 68s-1.
Material and methods
The study of the rheological behavior of chocolate was carried out with a rotary viscometer DV-3P which has a cylindrical adapter for the temperature sensor pt100 and TR9. Measurements were performed at temperatures between 36 and 44°C and shear rates between 0.1s-1 and 68s-1.
Results and discussion
Figure 1 shows the dependence of the dynamic viscosity on the shear rate at a temperature of 36°C (black curve). The red curve represents the polynomial fitting of the experimental data at a temperature of 36°C.
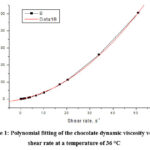 |
Figure 1: Polynomial fitting of the chocolate dynamic viscosity versus shear rate at a temperature of 36 °C |
Through the polynomial fitting of the chocolate dynamic viscosity versus shear rate at a temperature of 36 °C, we obtained the following rheological equation8-12:
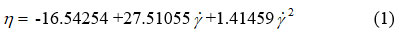
where: n – dynamic viscosity (Pas) and γ – shear rate (s–1).
Table 1 shows the rheological parameters obtained by polynomial fitting of the experimental data for chocolate in the temperature range of 36 and 44 °C.
Table 1: Constants equations (1)
|
Temperature, °C |
Constants equations (1) |
r2 |
||
| η0 |
a |
b |
||
|
36 |
-16.54254 |
27.51055 |
1.41459 |
0.99976 |
|
38 |
-9.98947 |
22.19128 |
1.37635 |
0.99996 |
|
40 |
-13.4235 |
23.04861 |
1.22829 |
0.99994 |
|
42 |
-12.94742 |
22.1533 |
1.13935 |
0.99994 |
|
44 |
-12.51043 |
21.58241 |
1.0596 |
0.99993 |
Figures 2, 3, 4, 5 and 6 show the second-order exponential fit of the dependence of dynamic viscosity on shear rate between temperatures 36 and 44°C.
 |
Figure 2: Second-order exponential fitting of the chocolate dynamic viscosity versus shear rate at 36 °C |
By second-order exponential fitting of the chocolate dynamic viscosity versus shear rate at a temperature of 36 °C, we obtained the following rheological equation:
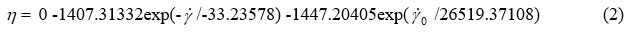
where: η – dynamic viscosity (Pas) and γ – shear rate (s–1).
Table 2 shows the rheological parameters obtained by second-order exponential fitting of the experimental data for chocolate in the temperature range of 36 and 44 °C.
Table 2: Constants equations (2)
|
Temperature, °C |
Constants equations (2) |
r2 |
||||
| η0 |
A |
B |
C |
D |
||
|
36 |
0 |
1407.31332 |
-33.23578 |
-1447.20405 |
26519.37108 |
0.99838 |
|
38 |
0 |
1186.95689 |
-31.66672 |
-1221.80369 |
3.79535E137 |
0.99889 |
|
40 |
0 |
1749.85184 |
-41.28381 |
-1804.29049 |
1.69326E90 |
0.99854 |
|
42 |
0 |
1665.26853 |
-41.65209 |
-1716.03993 |
1.01684E108 |
0.99854 |
|
44 |
0 |
1601.31463 |
-42.1315 |
-1648.64454 |
5.69166E105 |
0.99857 |
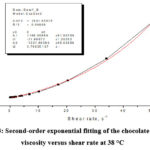 |
Figure 3: Second-order exponential fitting of the chocolate dynamic viscosity versus shear rate at 38 °C |
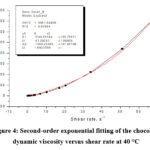 |
Figure 4: Second-order exponential fitting of the chocolate dynamic viscosity versus shear rate at 40 °C |
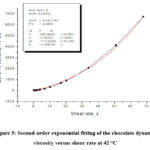 |
Figure 5: Second-order exponential fitting of the chocolate dynamic viscosity versus shear rate at 42 °C |
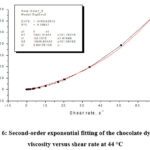 |
Figure 6: Second-order exponential fitting of the chocolate dynamic viscosity versus shear rate at 44 °C |
Conclusions
Chocolate was first manufactured in Italy by a confectioner from Florence who noticed that cocoa powder dissolves in water or milk. He then added vanilla and sugar.
The article proposes two rheological equations that describe the non-Newtonian behavior of chocolate at shear rates between 0.1s-1 and 68s-1 and temperatures between 36 and 44 °C.
References
- Gonçalves, E. V., & Lannes, S. C. D. S. (2010). Chocolate rheology. Food Science and Technology, 30, 845-851.
CrossRef - De Graef, V., Depypere, F., Minnaert, M., & Dewettinck, K. (2011). Chocolate yield stress as measured by oscillatory rheology. Food Research International, 44(9), 2660-2665.
CrossRef - Chevalley, J. (1991). An adaptation of the Casson equation for the rheology of chocolate. Journal of texture studies, 22(2), 219-229.
CrossRef - Gabriele, D., Migliori, M., Baldino, N., & De Cindio, B. (2008, July). Influence of fat content on chocolate rheology. In AIP Conference Proceedings (Vol. 1027, No. 1, pp. 1265-1267). American Institute of Physics.
CrossRef - Karnjanolarn, R., & McCarthy, K. L. (2006). Rheology of different formulations of milk chocolate and the effect on coating thickness. Journal of texture studies, 37(6), 668-680.
CrossRef - Vernier, F. C. (1997). Influence of emulsifiers on the rheology of chocolate and suspensions of cocoa or sugar particles in oil (Doctoral dissertation, The University of Reading).
- Efraim, P., Marson, G. C., Jardim, D. C., Garcia, A. O., & Yotsuynagi, K. (2011). Influence of phytosterols addition in the rheology and sensory attributes of dark chocolate. Procedia Food Science, 1, 1633-1637.
CrossRef - Rohm, H., Böhme, B., & Skorka, J. (2018). The impact of grinding intensity on particle properties and rheology of dark chocolate. LWT, 92, 564-568.
CrossRef - Stanciu I., 2019, Journal of Science and Arts, 3(48), 703-708.
- Stanciu I., 2019, Journal of Science and Arts, 4(49), 938-988.
- Stanciu I., 2011, Journal of Science and Arts, 1, 55-58.
- Stanciu I., 2018, Journal of Science and Arts, 18(2), 453-458

This work is licensed under a Creative Commons Attribution 4.0 International License.










