Impact of Successive Addition of Precursor in Synthesis of Copper and Silver Nanoparticles using the Tulsi Leaf Extract.
1Telangana Social Welfare Residential Degree College for Women, Nalgonda, Telangana State, India.
2Biophysics Research Laboratory, Department of Physics, Nizam College (Autonomous), Osmania University, Hyderabad, Telangana State, India.
3JNTUH, Kukatpally, Hyderabad, Telangana State, India.
Corresponding Author E-mail: kaleemjaleeli@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400216
Article Received on : 21 Feb 2024
Article Accepted on : 11 Apr 2024
Article Published : 24 Apr 2024
Reviewed by: Dr. Alyaa Munadi
Second Review by: Dr. Bhuvnesh Kumar Singh
Final Approval by: Dr. Dinesh Chand Sharma
Investigating the impact of successive introduction of precursor in making of silver-copper bimetallic green nanoparticles utilizing Tulsi leaf extract both as a stabilizing and reducing agent. Green synthesis methods using plants and plant-based extract are more beneficial and biodegradable than any other synthesis methods. Compared to other approaches plants generate nanoparticles which are more stable, and scaling them up is very easy. The impact of electronegativity of Ag and Cu metals on the formation and growth of these bimetallic nanoparticles has been studied. Energy dispersive X-ray analysis (EDX) and scanning electron microscopy (SEM) were used to investigate the biosynthesized Ag-Cu nanoparticles and Cu-Ag nanoparticles
KEYWORDS:Bimetallic; Electronegativity; Green synthesis Nanoparticles
Download this article as:| Copy the following to cite this article: Avula A, Jaleeli K. A, Sreekanth T. Impact of Successive Addition of Precursor in Synthesis of Copper and Silver Nanoparticles using the Tulsi Leaf Extract. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Avula A, Jaleeli K. A, Sreekanth T. Impact of Successive Addition of Precursor in Synthesis of Copper and Silver Nanoparticles using the Tulsi Leaf Extract. Orient J Chem 2024;40(2). Available from: https://bit.ly/4df9u4r |
Introduction
Nanotechnology which deals with materials at the nanoscale. It is used to formulate and engineer devices for many applications. The key components of nanotechnology are nanoparticles. These nanoparticles possess exceptional properties like surface to volume ratio, make nanoparticles chemically more sensitive and at particle size, less than 10nm optical and electronic properties differ due to quantum confinement. 1 Amalgamation of two different metal elements is known as bi-metallic nanoparticle which exhibit better properties and can be applied for innovative applications. Bimetallic are divided into types depending on how their atoms are organized, such as alloy, core-shell, intermetallic, and subcluster. The characteristics of a bimetallic nanoparticle depend on the metallic nanoparticles it includes. 2. In recent years, there has been a growing attention in bimetallic nanoparticles due to their physical structures, chemical, mechanical, thermal, optical, catalytic, and magnetic properties 3-7. The bimetallic nanoparticles offer more properties over monometallic nanoparticles 8-10. The bimetallic nanoparticles find applications in a wide array of fields, including nanomedicine, wastewater treatment, oil industry, agriculture, biosensors, gas industry, biomedical, drug delivery agents, imaging, food processing 11-17. Furthermore, extensive research has been carried to find the antimicrobial and anticancer efficiency of bimetallic nanoparticles. The conventional methods for synthesizing nanomaterials, like physical or chemical methods, tend to be expensive, labor-intensive, slow, environmentally hazardous, pose risks to human health. Hence, there is a need for environmentally friendly, efficient, clean, reliable, simple to scale up and inexpensive synthesis technique that can control these drawbacks. The natural synthesis method also the ‘green synthesis method,’ emerges as a substitute for synthesing nanomaterials. In this method the nanoparticles are synthesized using natural sources like plants or microorganisms, resulting in an end product that is environmentally friendly as well as biocompatible. Numerous types of nanoparticles, ranging from mono to quad metallic nanoparticles, have been successfully synthesized utilizing plant extracts. Nanoparticles produced through this approach are commonly referred to as biogenic nanoparticles or nanomaterials 3. In the biosynthesis of bimetallic nanoparticles using plant-based extract, is affected by several factors including the process kinetics, adsorption kinetics, the pH of the plant extract, length of incubation, temperature, the concentrations of both the plant extract and metal salts. During the synthesis method these factors play a significant role in determining the magnitude and morphology of the nanoparticles 18. Plant-mediated synthesis presents several advantages compared to microbial synthesis like it eliminates the necessity for a bacterial culture, fast process, occurs at room temperature and the plant extracts has various bioactive components serve as both reducing and stabilizing agents, this phenomenon is referred to as nano-phytotechnology. These bioactive components comprise proteins, carbohydrates, vitamins, amino acids, flavonoids, alkaloids, terpenoids, ketones, tannins, aldehydes, amides, polysaccharides, polyphenols, carboxylic acids, and phenolic acids 19,20. Copper-silver (Cu-Ag) nanoparticles were synthesized using an aqueous extract derived from date palm tree, synthesis process involved heating at 95°C with continuous stirring for a duration of 1 hour, resulting in the production of nanoparticles with an approximate size of 26 nanometers 21. Amaranthus tricolor, another Argo waste, was employed as both a reducing and stabilizing agent in the synthesis of Ag-Cu bimetallic nanoparticles22. Cu-Ag nanoparticles were synthesized using Kigelia Africana fruit as the precursor material, the synthesis technique was managed at a temperature of 120°C under reflux conditions 20,23. Aloe vera leaf extract was employed in the synthesis of Ag-Cu nanoparticles directly on cotton fabric, making it suitable for application as a wound dressing material24. The dimensions and shape of the particles are strongly dependent on the sequence in which the precursors are introduced. Herein, discovered the impact of successive precursor introduction on the size dimensions of Ag-Cu nanoparticles using Tulsi leaf extract. Tulsi or holy basil is “The Queen of Herbs,” rich in secondary metabolites like flavonoids, phenols etc. The Tulsi leaf extract is incorporated to the metal precursor solution containing the respective metal compounds to initiate the green synthesis of green nanoparticles. The possible reduction mechanism could be as follows.
M+ +e– (leaf extract) → Mo (M=Ag or Cu).
Materials
For experiments, Silver Nitrate (AgNO3), Copper (II) Sulphate pentahydrate (CuSO4 · 5H2O), were purchased from, Abids, India. Deionized water was provided the laboratory.25g of fresh Tulsi leaves were collected from botanical garden at Kondapur, Hyderabad, India.
Experimental Procedure
Tulsi Leaf Extract Preparation
25g of Tulsi leaves were washed thrice with distilled water to get rid of undesirable dust particles present on the Tulsi leaves. After washing Tulsi leaves were air dried and chopped, and were placed in a 250ml flask containing 100ml of deionized water. The temperature of the solution was raised to 40oC using magnetic stirrer.
Addition of CuSO4·5H2O Followed by AgNO3 in Tulsi Leaf Extract.
In this experiment, a colour change from light green to dark green was observed when Copper nanoparticles were primarily synthesized by introducing 0.01M CuSO4.5H2O solution into a 30 ml of Tulsi leaf extract. Using a magnetic stirrer the solution was agitated for one minute. Subsequently, 0.01M silver nitrate solution was incorporated into the solution containing copper nanoparticles, and the mixture was further heated and stirred. The progression of Cu-Ag nanoparticle growth was then examined using SEM and EDX analysis. The resulting particles were denoted as Cu-Ag nanoparticles.
Addition of AgNO3 Followed by CuSO4·5H2O in Tulsi Leaf Extract.
In this method, we introduced a modification in the sequence of precursor addition. Initially, a solution of 30 ml of Tulsi leaf extract was combined with 0.01 M AgNO3 solution, a variation in the color of the solution from transparent to brown colour was observed indicating generation of silver (Ag0) nanoparticles from silver ions (Ag+). Following this, .01M copper sulfate solution was incorporated into the solution containing silver nanoparticles and heated for 1hr using a mag netic stirrer. The resultant particles were named as Ag-Cu nanoparticles. Subsequently, SEM and EDX were employed to investigate the characteristics of Ag-Cu particles.
Characterization
SEM
Scanning Electron Microscopy (SEM) plays a crucial role., while analyzing the green nanoparticles. Scanning Electron Microscopy is an advanced microscopy technique that employs a beam of electrons to scan the surface of a specimen. It provides highly magnified, three-dimensional pictures, allowing researchers to study the morphology, size, and dispersal of nanoparticles.
EDX
EDX is a multipurpose tool in nanoparticle synthesis and characterization, providing essential information about the elemental composition and distribution within materials. It is instrumental in verifying the success of synthesis processes and ensuring the desired properties of nanoparticles for various applications.
Results and Discussion
Possible Bio-Reduction Mechanism for Synthesis of the Green Nanoparticles.
There are three basic stages in the bio-reduction mechanism of metal nanoparticles in plants and plant extracts 25. The first stage is activation, during which cations in the metal salt are reduced into metal atoms and are nucleated. The two stages are the growth stage, where metals atoms move arbitrarily and colloid with cations, results in the creation of clusters. At this stage, starting concentration of precursors is controlled because nuclei generation must grow quicker and slower progress of nuclei. The nucleus is formed through continuous collision of clusters, atoms and cations, and the termination stage, nucleus grows to a critical size and lastly formation of nanoparticle.
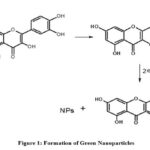 |
Figure 1: Formation of Green Nanoparticles. |
From Fig.126 when metal salts are dissolved in distilled water, splits into metal ions. These metal ions interact with the Tulsi leaf extract which contains high number of flavonoids and a charge transfer occurs between metal ion and flavonoids results in the formation if capped nanoparticles.
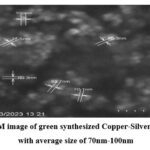 |
Figure 2: SEM image of green synthesized Copper-Silver nanoparticles with average size of 70nm-100nm. |
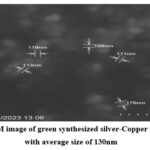 |
Figure 3: SEM image of green synthesized silver-Copper nanoparticles with average size of 130nm |
SEM image (Fig.2), the observed nanoparticles demonstrate a size range of approximately 70-100nm. Furthermore, SEM image (Fig.3) provides additional confirmation of the presence of larger Ag-Cu particles, measuring around 90-150nm. This designates the importance of precursor addition sequence on the formation of green nanoparticles. The distinction between Ag-Cu nanoparticles and Cu-Ag nanoparticles can be attributed to the process kinetics employed in the synthesis procedures. In the synthesis procedure of Cu-Ag nanoparticles, silver nitrate reduces to silver nanoparticles not only due bio components of Tulsi leaf extract but also experience reduction due to galvanic displacement reaction of copper ions. This implies that, Cu actively helps not only in the reduction of Ag, and but also in the nucleation. Conversely, in the synthesis of Ag-Cu nanoparticles, the Ag nanoparticles formed do not participate in the reduction of Cu ions and do not contribute in nucleation process. Subsequently, the particles formed in this scenario are larger in size compared to those in the Cu-Ag synthesis. These conclusions emphasize the critical role of precursor addition sequence in nanoparticle synthesis procedure. Also, this agrees with the previously published study results 27.
EDX
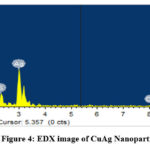 |
Figure 4: EDX image of CuAg Nanoparticles |
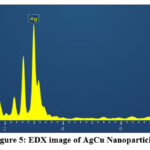 |
Figure 5: EDX image of AgCu Nanoparticles. |
The EDX examination substantiates that the Cu-Ag nanoparticles from (Fig.4) consist of 43.94 wt% Ag and 43.95 wt% Cu. Signals Sulphur presences possibly due to the presence of phytochemicals on the surface of Ag-Cu NPs’, were observed. From (Fig .5) Ag-Cu nanoparticles consists of 88.38 wt% Ag and 9.51 wt% Cu. The presences of oxygen is attributed to phytochemicals present in the Tulsi leaf extract.
Conclusion
Using Tulsi leaf extract as reducing agent, we investigated the impact of successive introduction of CuSO4.5H2O and AgNO3 as precursors. From the reports, it was found that due to electronegativity of the two metallic elements the size of final particles formed depends on the order of introduction of precursor in the synthesis of green nanoparticles. This green synthesis method can be applied to synthesize other bimetallic nanoparticles. Further studies are required to test the anti-bacterial effect and efficiency of synthesized silver-Copper and Copper-Silver nanoparticles.
References
- Idris DS, Roy A.Crystals. 2023; 13(4):637. https://doi.org/ 10.3390/cryst13040637.
CrossRef - Mazhar, T.; Shrivastava, V.; Tomar, R.S. J. Pharm. Sci. Res. 2017, 9, 102–110.
- Behera, A.; Mittu, B.; Padhi, S.; Patra, N.; Singh, J.Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Micro and Nano Technologies; Elsevier, 2020; 25,639–682.
CrossRef - Saleh, T.A. Environ. Technol. Innov. 2020, 20, 101067.
CrossRef - Wu, Q.; Miao, W.; Zhang, Y.; Gao, H.; Hui, D. Nanotechnol. Rev. 2020, 9, 259–273.
CrossRef - Liu, X.; Wang, D.; Li, Y. Nano Today 2012, 7, 448–466.
CrossRef - Shamsiev, R.S.; Danilov, F.O.; Flid, V.R. Russ. Chem. Bull. 2022, 71, 220–226.
CrossRef - Arora, N.; Thangavelu, K.; Karanikolos, G.N. Front. Chem. 2020, 8, 412.
CrossRef - Duan, M.; Jiang, L.; Zeng, G.; Wang, D.; Tang, W.; Liang, J.; Wang, H.; He, D.; Liu, Z.; Tang, L., Appl. Mater. Today 2020, 19, 100564.
CrossRef - Hu, D.; Xu, H.; Yi, Z.; Chen, Z.; Ye, C.; Wu, Z.; Garces, H.F.; Yan, K. ACS Sustain. Chem. Eng. 2019, 7, 15339–15345.
CrossRef - Stephanie, R., Kim, M. W., Kim, S. H., Kim, J. K., Park, C. Y., & Park, T. J. TrAC – Trends in Analytical Chemistry,2021, 135, Article 116159. https://doi.org/10.1016/j.trac.2020.116159
CrossRef - McNamara, K.; Tofail, S.A.M. Adv. Phys. X 2016, 2, 54–88.
CrossRef - Scaria, J.; Nidheesh, P.V.; Kumar, M.S. J. Environ. Manag. 2020, 259, 110011.
CrossRef - Khalil, M.; Jan, B.M.; Tong, C.W.; Berawi, M.A. Appl. Energy 2017, 191, 287–310.
CrossRef - Basavegowda, N.; Mandal, T.K.; Baek, K.-H. Food Bioprocess Technol. 2020, 13, 30–44.
CrossRef - Srinoi, P.; Chen, Y.-T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Appl. Sci. 2018, 8, 1106.
CrossRef - Pissuwan, D.; Niidome, T.; Cortie, M.B. J. Control. Release 2011, 149, 65–71.
CrossRef - Vijayaraghavan, K.; Ashokkumar, T. J. Environ. Chem. Eng. 2017, 5, 4866–4883.
CrossRef - Lateef, A.; Ojo, S.A.; Folarin, B.I.; Gueguim-Kana, E.B.; Beukes, J. Clust. Sci. 2016, 27, 1561–1577.
CrossRef - Providence, B.A.; Chinyere, A.A.; Ayi, A.A.; Charles, O.O.; Elijah, T.A.; Ayomide, H.L. Int. J. Phys. Sci. 2018, 13, 24–32.
CrossRef - Al-Haddad, J.; Alzaabi, F.; Pal, P.; Rambabu, K.; Banat, F. Clean Technol. Environ. Policy 2020, 22, 269–277
CrossRef - Chaturvedi, V.K.; Yadav, N.; Rai, N.K.; Bohara, R.A.; Rai, S.N.; Aleya, L.; Singh, M.P. Environ. Sci. Pollut. Res. 2021, 28, 13761–13775.
CrossRef - Mohanlall, V.; Biyela, B.Indian J. Biochem. Biophys. (IJBB) 2022, 59, 94–102
CrossRef - Mamatha, G.; Varada Rajulu, A.; Madhukar, K. J. Nat. Fibers 2020,17,1121
CrossRef - Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME, Kalinina NO Acta Naturae, 2014,6(1):35–44
CrossRef - Jain, S., & Mehata, M. S. Scientific Reports. 2017 ,7, 15867 https://doi.org/10.1038/s41598-017-15724-8
CrossRef - K. V. Kinhal, N. P. Bhatt and S. Pushpavanam, 2017 IEEE 12th Nanotechnology Materials and Devices Conference (NMDC), 171-172, doi: 10.1109/NMDC.2017.8350542.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










