Highly Effective Antifungal and Antibacterial Properties of ZnO, ZnS, FeS2 and SnO2 Nanoparticles Against Various Fungal and Bacterial Isolates
Nano Lab, School of Sciences ITM University Gwalior MP India.
Corresponding Author E-mail: ycgoswami@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400222
Article Received on : 05 Feb 2024
Article Accepted on :
Article Published : 22 Apr 2024
Reviewed by: Dr. B. C. Revanasiddappa
Second Review by: Dr. Amir Hassan
Final Approval by: Dr. Abdel Omri
This study explores the potential of four types of nanoparticles (ZnO, ZnS, FeS2, and SnO2) to combat resistant microbes using the well method. The research focuses on their antifungal and antibacterial properties. Results showed that FeS2 and ZnO nanoparticles displayed broad-spectrum activity against various bacteria and fungi. This was evident by the formation of clear inhibition zones after 24 hours at 37°C. These findings highlight the promise of FeS2 and ZnO nanoparticles as weapons against resistant microbes. The inhibition zones demonstrate a measurable effect on microbial growth, providing valuable groundwork for further development of novel strategies to fight and manage microbial infections. This research adds to the ongoing search for alternative and effective solutions in the face of growing microbial resistance.
KEYWORDS:Antibacterial properties; Antimicrobial properties; Metal Oxides; Metal Sulphides; Nanoparticles; Zinc Sulphides; Zinc Oxides
Download this article as:| Copy the following to cite this article: Goswami Y. C, Goswami R, Chirova T. K. Highly Effective Antifungal and Antibacterial Properties of ZnO, ZnS, FeS2 and SnO2 Nanoparticles Against Various Fungal and Bacterial Isolates. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Goswami Y. C, Goswami R, Chirova T. K. Highly Effective Antifungal and Antibacterial Properties of ZnO, ZnS, FeS2 and SnO2 Nanoparticles Against Various Fungal and Bacterial Isolates. Orient J Chem 2024;40(2). Available from: https://bit.ly/4cZtaJg |
Introduction
The emergence of antibiotic-resistant bacteria and fungi presents a significant challenge in healthcare. Widespread and often inappropriate use of antibiotics has fueled this resistance, rendering many existing treatments ineffective. This highlights the urgent need for novel antimicrobial solutions. Nanoparticles, particles typically between 1-100 nanometers in size, have emerged as a promising avenue in this fight. Their unique properties offer potential advantages over traditional antibiotics. Studies have shown nanoparticles to possess antimicrobial, anti-inflammatory, and wound-healing properties6, 7. However, this field is still relatively young, and research on nanoparticle safety is ongoing.
A key concern with nanoparticles is their toxicity. Their small size, beneficial in many applications, can also increase their reactivity, potentially leading to harmful effects. Various factors influence toxicity, including size, surface area, and chemical composition 8, 9. These factors can interact, with combinations potentially leading to higher toxicity levels. Studies have shown a complex relationship between nanoparticle concentration and toxicity. In some cases, higher concentrations may lead to reduced toxicity due to particle aggregation, hindering cellular uptake 10. However, smaller nanoparticles, with easier cellular entry, may exhibit higher toxicity 11,12.
Despite these concerns, research suggests potential for safe and effective use of nanoparticles as antimicrobials. Green-synthesized ZnO nanoparticles have shown promise in combating bacterial and fungal pathogens 6. However, other studies have highlighted the potential toxicity of nanoparticles towards non-targeted organisms 14.
This study focuses on evaluating the antifungal and antibacterial activity of four specific nanoparticles: ZnO, ZnS, FeS2, and SnO2. We aim to assess their effectiveness against various fungal and bacterial isolates. The study will also investigate the influence of concentration on their activity. By exploring these aspects, we hope to contribute valuable knowledge to the development of safe and effective nanoparticle-based antimicrobials.
Materials and Methods
Materials
Fungal and Bacterial Isolates
Fungal isolates (Aspergillus niger and Aspergillus fumigatus) and bacterial isolates (Escherichia coli, Bacillus cereus, and Bacillus subtilis) were obtained from the Microbiology lab. These isolates were stored at 4°C and revived by culturing them on Sabouraud Dextrose Agar (SDA) or Nutrient Agar (NA) at 37°C for 24 hours.
Methods: Nanoparticle Synthesis
A modified hydrothermal method with dual precipitation and ultrasonication was used to synthesize SnO2, ZnS, FeS2, and ZnO nanoparticles.
The synthesis involved following steps:
Step 1: Preparation of precursor solutions: Separate solutions of 0.1 M ZnSO4·7H2O and 0.3 M Na2S were prepared in 10 mL DI water with stirring.
Step 2: SnO2 synthesis: 0.1 M SnCl2·2H2O was dissolved in a mixture of 20 mL DI water and 20 mL ethanol. After adding 0.1 M NH4NO3, the solution was stirred for 30 minutes at 50°C.
Antifungal Activity Assay
Sabouraud Dextrose Agar (SDA) media was prepared (100 mL) and autoclaved (121°C, 15 psi for 15 min). Six Petri dishes were also autoclaved.The sterilized media was poured into the Petri dishes and allowed to solidify within a Laminar Airflow Cabinet.Using a sterile 6 mm borer, two wells were created on opposite sides of the solidified media in four plates.Two of these plates were inoculated with Aspergillus niger and the other two with Aspergillus fumigatus using a spreader for even distribution.One plate served as the positive control (inoculated with fungi only) and another as the negative control (no fungi).The experiment evaluated the impact of synthesized nanoparticles on fungal growth.
Antibacterial Activity Assay
Nutrient Agar media (100 mL) was prepared and autoclaved along with six Petri dishes (121°C, 15 psi for 15 min). The media was then poured and allowed to solidify in the plates. Similar to the antifungal assay, wells were created in four plates using a sterile 6 mm borer. Bacterial suspensions were prepared for E. coli, Bacillus subtilis, and Bacillus cereus by suspending bacteria in sterilized water. Each suspension was spread on separate plates using a sterile spreader. One plate served as the positive control (inoculated with bacteria) and another as the negative control (no bacteria). Varying concentrations (15 µL, 20 µL, 25 µL, and 30 µL) of each nanoparticle (ZnO, FeS2, SnO2, or ZnS) were loaded into one well per plate using a micropipette. An equal volume of ethanol was loaded into the opposite well as a control. The plates were incubated at 37°C for 24 hours to allow bacterial growth. After incubation, the plates were examined for zones of inhibition around the wells containing nanoparticles or ethanol. The diameter of any inhibition zone was measured and recorded. The entire antibacterial activity assay was repeated three times for each bacteria-nanoparticle combination to ensure reliable results.
The modified hydrothermal method with dual precipitation and ultrasonication synthesised the SnO2, ZnS, FeS, ZnO nanocomposite. The synthesis process involved three main steps.In the first step, separate 0.1M ZnSO4 7H2O and 0.3M Na2S aqueous solutions were prepared in 10mL DI water separately with continuous stirring. The presence of ZnS nanoparticles in the resulting milky white solution was confirmed using UV-vis spectroscopy.
For SnO2, 0.1M SnCl2 2H2O was dissolved in a mixture of 20mL DI water and 20mL ethanol, followed by the addition of 0.1M NH4NO3. The solution was stirred for 30 minutes at 50°C, resulting in a milky white solution containing SnO2 nanoparticles, which was confirmed using UV-vis spectroscopy. Characterization of these nanoparticles was carried out with a reported concentration of 0.5M in 50cc of ethanol 19-22.
Results and Discussion
Antifungal Studies
The experiment evaluated the antifungal activity of synthesized nanoparticles (FeS2, ZnO, SnO2, and ZnS) against Aspergillus niger. Zone of inhibition formation and diameter were used to assess efficacy. Diameter of zone of inhibition results produced by nanoparticles at varying concentrations against A. niger shown in Fig.1.
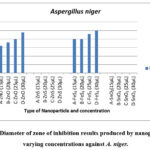 |
Figure 1: Diameter of zone of inhibition results produced by nanoparticles at varying concentrations against A. niger. |
Figure 1 shows that FeS2 and ZnO nanoparticles consistently produced inhibition zones against A. niger at all tested concentrations. In contrast, SnO2 and ZnS did not exhibit any inhibitory effect at any concentration. The presence of inhibition zones indicates potential fungicidal activity, possibly linked to reactive oxygen species (ROS) production, which aligns with previous research 6.
The diameter of the inhibition zone increased with increasing concentrations of FeS2 and ZnO, suggesting a dose-dependent antifungal effect. Both nanoparticles impeded fungal growth near the loaded wells. Interestingly, FeS2 displayed stronger antifungal activity compared to ZnO. At the lowest concentration (15 µL), FeS2 produced a 20 mm zone of inhibition compared to ZnO’s 16 mm. This trend continued at higher concentrations (25 mm for FeS2 vs. 24 mm for ZnO at 30 µL). Notably, FeS2 exhibited the same inhibition zone diameter (20 mm) at 15 µL and 20 µL concentrations, suggesting a possible plateau effect, where further concentration increase may not significantly enhance antifungal activity. This aligns with the concept of dose-dependent nanoparticle toxicity 25.
Our findings contradict observations by23 who suggested that higher concentrations might not be effective due to aggregation. In contrast, this study demonstrates increased toxicity of FeS2 and ZnO at higher concentrations. Conversely, SnO2 and ZnS lacked any inhibitory effect, potentially indicating lower A. niger susceptibility or limited nanoparticle toxicity against this fungus. This difference in inhibition zones between ZnO and FeS2 highlights the varying susceptibility of fungi to different nanoparticle types 25-27.
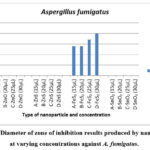 |
Figure 2: Diameter of zone of inhibition results produced by nanoparticles at varying concentrations against A. fumigatus. |
As depicted in Figure 2, the results highlight FeS2 nanoparticles as the sole contributor to antifungal activity against A. fumigatus, evident in the production of zones of inhibition (as illustrated in Figure 3). In contrast, ZnS, SnO2, and ZnO nanoparticles yielded negative results, failing to produce distinct zones of inhibition. This absence of clear zones may suggest that A. fumigatus, as a fungal species, was less susceptible to the toxic effects exerted by the nanoparticles.
Examining Figure 2 further, it is noted that FeS2 at concentrations of 15 µL and 20 µL displayed identical zone of inhibition diameters of 14 mm, indicating that an increase from 15 µL to 20 µL did not enhance antifungal activity. This similarity mirrors the anomaly observed in the results against A. niger, suggesting that this concentration range may not significantly affect the activity against the fungi. However, FeS2 exhibited smaller zones of inhibition against A. fumigatus compared to A. niger, suggesting a potential variation in sensitivity or resistance between the two fungal strains. 17 emphasized that the toxic effects of nanoparticles on microorganisms depend not only on the nanoparticle type but also on the microbial species involved, which aligns with the observed differences in sensitivity.
FeS2′ antifungal efficacy against A. fumigatus (with a maximum zone of inhibition of 20 mm) was less pronounced than its impact on A. niger (with a minimum zone of inhibition of 20 mm). This disparity may indicate that A. fumigatus is a more resistant strain, producing smaller zones of inhibition. This inference is reinforced by the observation that ZnO, effective against A. niger, only managed to restrict the growth of A. fumigatus near the well, without clear zones of inhibition. This discrepancy suggests the possibility that increasing the concentration of ZnO beyond 30 µL might be necessary for the production of distinct zones of inhibition.
It is noteworthy that the wells loaded with ethanol did not hinder fungal growth, signifying that all observed antifungal activity is solely attributed to the nanoparticles. In summary, with regard to antifungal activity, FeS2 demonstrated superior efficacy, followed by ZnO nanoparticles. The mechanism of action involves the disruption of the cell membrane, leading to cellular disruption and death, coupled with the production of radical oxygen species that are detrimental to cell organelles 6, 2,23. Furthermore, the maintenance of inhibition zones after 48 hours indicates the sustained effectiveness of nanoparticles in their fungicidal activity, also reflecting proper diffusion within the agar media.
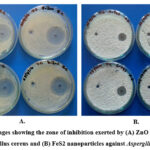 |
Figure 3: Images showing the zone of inhibition exerted by (A) ZnO nanoparticles against Bacillus cereus and (B) FeS2 nanoparticles against Aspergillus fumigatus |
Antibacterial Studies
Figure 3 demonstrates the formation of clear inhibition zones around wells containing ZnO and FeS2 nanoparticles against Bacillus cereus. This observation suggests the nanoparticles’ ability to diffuse through the media and inhibit bacterial growth, potentially indicating bactericidal activity 6. The presence of inhibition zones aligns with previous research highlighting the nanoparticle’s potential to impede bacterial growth.
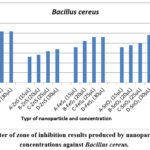 |
Figure 4: Diameter of zone of inhibition results produced by nanoparticles at varying concentrations against Bacillus cereus. |
Figure 4 shows the concentration-dependent antibacterial activity of ZnO, FeS2, SnO2, and ZnS against Bacillus cereus. ZnO consistently produced the largest inhibition zones at all tested concentrations, followed by SnO2, FeS2, and ZnS. Notably, ZnO exhibited a larger zone (25 mm) at 20 µL compared to the highest concentration of ZnS (17 mm), SnO2 (24 mm), and FeS2 (23 mm). ZnO reached its maximum zone size (26 mm) at 30 µL, while ZnS had the smallest zone (17 mm) at the same concentration.
Interestingly, all nanoparticles except ZnO and FeS2 displayed increasing inhibition zones with higher concentrations. This aligns with the concept of concentration-dependent nanoparticle toxicity 6,17,30.
The observed variations in bacterial growth inhibition support previous findings 17 that nanoparticle toxicity depends on both nanoparticle type and bacterial species. After 48 hours, clear inhibition zones remained for ZnO, ZnS, and FeS2, while SnO2 showed bacterial growth within the zone. This could be due to a decrease in SnO2 nanoparticle concentration as bacterial cells divided, reducing the observed antibacterial effect 31,32.
Furthermore, Bacillus cereus demonstrated higher susceptibility to ZnO and lower susceptibility to ZnS, highlighting species-specific sensitivity to nanoparticles, as reported by 17.
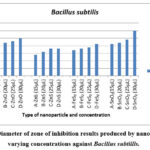 |
Figure 5: Diameter of zone of inhibition results produced by nanoparticles at varying concentrations against Bacillus subtillis. |
Figure 5 demonstrates the concentration-dependent antibacterial activity of ZnO, FeS2, SnO2, and ZnS against Bacillus subtilis. SnO2 displayed the largest inhibition zones for all concentrations except 20 µL, where it matched ZnO and FeS2 and showed moderate effectiveness, while ZnS had the weakest effect. Notably, SnO2 consistently exhibited larger inhibition zones compared to other nanoparticles.
Maximum inhibition for SnO2 was observed at 30 µL (30 mm zone), while ZnS had the smallest zone (18 mm) at the same concentration. All nanoparticles displayed increasing inhibition zones with higher concentrations, aligning with the concept of concentration-dependent nanoparticle toxicity 6,17,31.
Similar to B. cereus, these findings suggest bacterial species and nanoparticle type influence toxicity 17. B. subtilis showed varying sensitivity, with SnO2 being the most effective, followed by ZnO, FeS2, and ZnS.
After 48 hours, clear inhibition zones remained for ZnO, ZnS, and FeS2, while SnO2 showed bacterial growth within the zone. This might be due to a decrease in SnO2 nanoparticle concentration as bacteria divided, reducing the observed effect 32,33.
In conclusion, SnO2 exhibited the strongest antibacterial activity against B. subtilis, followed by ZnO, FeS2, and ZnS. This study highlights the importance of considering both nanoparticle type and bacterial species for effective antibacterial applications.
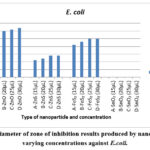 |
Figure 6: Diameter of zone of inhibition results produced by nanoparticles at varying concentrations against E.coli. |
Figure 6 presents the outcomes of the assessment of test nanoparticles against E. coli. Results indicated that all nanoparticles, with the exception of SnO2, were able to generate inhibition zones against E. coli. The most notable inhibition zone was observed for ZnO nanoparticles at 30 µL, displaying a 32 mm inhibition zone. Following ZnO, FeS2 exhibited a 25 mm inhibition zone, and ZnS showed a 14 mm inhibition zone at the same concentration for those nanoparticles that produced an inhibition zone. ZnO emerged as the most effective against E. coli, consistently producing the largest inhibition zones for all concentrations compared to the other test nanoparticles.
Consistent with prior research 16,17 and supported by 30, which emphasizes the concentration-dependent nature of nanoparticle toxicity, the results for E. coli in this study aligned with the notion that inhibition zones tend to increase with higher concentrations. This pattern was observed for all test nanoparticles except SnO2, which did not produce any inhibition zone for all concentrations. This finding is in line with 17, indicating that each species possesses a specific susceptibility to certain nanoparticles, and in the case of E. coli, it demonstrated insensitivity or resistance to SnO2 nanoparticles.
The results highlighted the heightened sensitivity of E. coli to ZnO nanoparticles, and all test nanoparticles maintained clear inhibition zones even after 48 hours of culture. This persistence underscores the sustained antibacterial effectiveness of the nanoparticles against E. coli.
Key Findings
Antibacterial Activity
ZnO nanoparticles exhibited the strongest and most consistent antibacterial effect across all bacterial isolates (Bacillus cereus, Bacillus subtilis, and E. coli). This is likely due to ZnO’s ability to disrupt bacterial cell membranes, leading to cell death. Their small size facilitates membrane penetration, causing stress and breakdown. FeS2 nanoparticles also displayed consistent antibacterial activity against all isolates, potentially through the generation of reactive oxygen species that damage bacterial cells. ZnS nanoparticles showed moderate and consistent antibacterial activity, while SnO2 was only effective against Bacillus species. This highlights the dependence of nanoparticle toxicity on both nanoparticle type and bacterial species. Bacillus cereus was the most susceptible bacteria, followed by Bacillus subtilis and then E. coli. This aligns with previous research suggesting E. coli’s lower susceptibility to nanoparticles compared to Bacillus subtilis.
Antifungal Activity
FeS2 nanoparticles were the most effective against the fungal isolate (Aspergillus niger), followed by ZnO nanoparticles. Both inhibited fungal growth by creating clear inhibition zones. Their mechanism of action likely involves disrupting fungal cell membranes and producing cell-damaging radical oxygen species. Importantly, the inhibition zones remained after 48 hours, indicating sustained fungicidal activity SnO2 and ZnS did not exhibit any antifungal activity against A. niger. This study supports the concept that nanoparticle effectiveness depends on both the type of nanoparticle and the specific microorganism (bacteria or fungi) being targeted Bacteria displayed greater overall susceptibility to the tested nanoparticles compared to fungi.
Conclusions
This study investigated the antibacterial and antifungal activity of synthesized ZnO, FeS2, SnO2, and ZnS nanoparticles. ZnO nanoparticles demonstrated the strongest and most consistent antibacterial effect against various bacteria (Bacillus cereus, Bacillus subtilis, and E. coli). FeS2 nanoparticles also displayed broad-spectrum antibacterial activity. Fungal susceptibility was lower compared to bacteria. FeS2 nanoparticles exhibited the most effective antifungal activity against Aspergillus niger, followed by ZnO nanoparticles. SnO2 and ZnS lacked antifungal properties against this fungus. Both bacterial and fungal susceptibility varied depending on the nanoparticle type, highlighting the importance of considering this factor in developing nanoparticle-based antimicrobial applications. This study provides valuable insights into the potential of these nanoparticles for developing targeted antimicrobial agents. Further research is necessary to ensure their safe and effective application.
Acknowledgements
The authors would like to express sincere gratitude to MPCST Bhopal, Nano Lab, and PC Ray Lab for sophisticated instrumentation,ITM University for providing the necessary facilities and resources for conducting the experiments and research activities.
Conflict of interest
There are no conflict of interest.
References
- Vaseem, M.; Umar, A.; Yoon-Bong, H. Am. Sci. Pub. 2010, 5, 1-36
- Bisauriya, R.; Verma, D.; Goswami, Y. C.; J. Mater. Sci: Mater Electron. 2018, 29, 1868–1876
CrossRef - Kumar, N.; Purohit, L.P.; Goswami, Y.C.; AIP Conf. Proc. 2015, 1675, 020030
- Liu, W. J. Biosci. Bioeng. 2006, 102,11-7
CrossRef - Sungkaworn, T.; Triampo, W.; Nalakarn, P.; Triampo, D.; Tang, I.M.; Lenbury Y. Int. J. Biomed. Sci. 2007, 2, 67-74
- Kumar, N.; Purohit, L.P.; Goswami, Y.C. Chal. Lett. 2015, 12, 333-338.
CrossRef - Kumar, N.; Pathak, T.K.; Purohit L.P.; Swart, H.C.; Goswami, Y.C.; Nano-Struct. Nano-Objects 2018, 16, 1-8
CrossRef - Hajipour M.; Fromm K.; Ashkarran, A.K.; Aberasturi, D. J.; Larramendi, I.; Rojo, T.; Serpooshan, V.; Parak, W.; Mahmoudi, M. Trends in Biotech. 2012, 30, 499-511
CrossRef - Vandeputte, P.; Ferrari S.; Coste, A. Int. J. Microbio. 2012, 2012,1-26
CrossRef - Gunalan, S.; Sivaraj R.; Rajendran, V., Progress in Nat. Science: Mater. Internat., 2012 22, 693-700
CrossRef - Taylor, P.L.; Ussher, A.L.; Burrell, R.E. Biomaterials, 2005, 26, 7221-7729
CrossRef - Merlin, J. P.; Xiaogang L. Front. Gen. 2022, 12, 10.3389/ fgene.2021.817974
- Buzea, A.; Pacheco, I.; Robbie, K. Biointerphases, 2007, 2, MR17
CrossRef - Gurr, J.; Wang, A.; Chen, C.; Jan; K. Toxicol. 2005, 213, 66-73
CrossRef - Kumar, V.; Rajaram, P.; Goswami, Y.C. J. Mat. Sci.: Mat. Elect., 2017 28, 9024-9031
CrossRef - Goswami, Y.C.; Mohapatra, R.; Kaundal, J.B. Chalcogenide letters 2021, 18, 255-262
CrossRef - Sharma, R.; Singh, R.; Goswami, Y.C.; Kumar V.; Kumar, D.; J. Aust. Ceram. Soc. 2021, 57, 697-703
CrossRef - Kumar, N.; Purohit, L. P.; Goswami, Y. C.; Physica E: Low-dim. Sys. Nano. 2017, 83, 333-338.
CrossRef - Goswami, Y.C.; Kumar, V.; Rajaram, P. Mat. Lett. 2014, 128, 425-428.
CrossRef - Lakshminarayanan, V.; Bhattacharya, I. Advances in Optical Science and Engineering, Springer Proceedings in Physics 2014,166, 533-539
- Nagaich, S.; Goswami, Y.C.; Fifth International Conference on Advanced Computing & Communication, 2015, 5,165-168 doi: 10.1109/ACCT.2015.16
CrossRef - N Kumar, N LP Purohit, L.P. YC Goswami, Y. C. Physica E: Low-dim. Sys. Nano. 2016, 83, 333-338
CrossRef - Singh, R.; Kumar, D.; Goswami, Y.C.; Sharma; R. Arab. J. Chem. 2019, 12, 1537-1544
CrossRef - Kumar, N.; Purohit, L.P.; Goswami Y. C. AIP Conf. Proc. 2015, 1675, 020030
- Sharma, R.; Singh, B.; Kumar, V.; Goswami, Y. C.; Singh, R.; Kumar, D. Adv. Opt. Sci. Eng. 2015 575-580
CrossRef - Takenaka, S.; Karg, E.; Roth, C.; Schulz, H.; Ziesenis, A.; Heinzmann, U.; Schramel P.; Heyder, J. Env. Health Persp. 2001, 109, 547
CrossRef - Oberdörster, G. ; Oberdörster, E.; Oberdörster, J. Env. Health Persp., 2005 113, 823-839
CrossRef - Jones, N.; Ray, B.; Ranjit, B.K.; Manna, A. FEMS Microbio. Lett., 2008, 279, 71-76
CrossRef - Aruoja, V.; H. Dubourguier, H.; Kasemets K.; Kahru, A., Sci. Total Env. 2009, 407, 1461-1468
CrossRef - Baek Y.; An, Y.; Sci. Total Env. 2011 409, 1603-1608
CrossRef - Brayner, R.; Ferrari-Iliou, R.; Brivois, R.N.; Djediat, S. Benedetti M. Fiévet, F. Nano Lett. 2006, 6, 866-870
CrossRef - Padmavathy N.; Vijayaraghavan, R. Sci. Tech. Adv. Mat. 2008, 9035004
- Huang, Z.; Zheng, X.; Yan, D.; Yin, G.; Liao, X.; Kang, Y.; Yao, Y.; Huang, D.; Hao, B. Langmuir 2008, 24, 4140-4144
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










